Abstract
Standard therapy has not been established for thyroid cancer when a thyroidectomy is contraindicated due to systemic disease. Herein, we reported a patient who had hypertrophic cardiomyopathy and papillary thyroid carcinoma treated by radiofrequency ablation because of inability to tolerate a thyroidectomy. Radiofrequency ablation can be used to treat thyroid cancer when surgery is not feasible, although the long-term outcome needs further observation.
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer. Although surgery is recommended for PTC, there is no consensus on the therapy when a thyroidectomy is contraindicated because of systemic disease such as heart failure or chronic obstructive pulmonary disease. Hypertrophic cardiomyopathy is a common genetic cardiovascular disease with a high risk of sudden death and disability (1). Given the potential devastating perioperative cardiac complications, hypertrophic cardiomyopathy is generally a contraindication for anesthesia and surgery (2). Radiofrequency ablation (RFA) has been used widely in the treatment of solid cancers (3). There is no report of PTC treated with RFA instead of surgery. Herein, we presented a case of PTC in a patient with hypertrophic cardiomyopathy treated with RFA.
Neck ultrasound performed for a 52-year-old female revealed a predominantly solid nodule (0.9 × 0.7 × 0.5 cm) with marked hypoechogenicty, microcalcification, moderate vascularity, and spiculated margin in the right thyroid gland. Enlarged neck lymph nodes were not detected (Fig. 1A, B). Total thyroxine (TT4) and total triiodothyronine (TT3) were 109.60 nmol/L (55.47–161.25 nmol/L) and 1.16 nmol/L (1.02–2.96 nmol/L); free thyroxine (FT4) and free triiodothyronine (FT3) were 14.63 pmol/L (10.45–24.38 pmol/L) and 4.36 pmol/L (2.77–6.31 pmol/L), respectively. Thyroid-stimulating hormone (TSH) and thyroid peroxidase antibody (TPO-Ab) were 1.58 mIU/L (0.380–4.340 mIU/L) and 852.0 IU/mL (0–100 IU/mL), respectively.
Seven years earlier, the patient had been diagnosed with hypertrophic cardiomyopathy and a DDD cardiac pacemaker was implanted. Her cardiac function was graded as New York Heart Association class III. A cardiac ultrasound showed diffuse thickening of the ventricular walls and the left ventricular ejection fraction was 60%. The N-terminal pro-brain natriuretic peptide was 1048 pg/mL (0–100 pg/mL).
Core needle biopsy (18 G needle) confirmed the diagnosis of PTC (Fig. 1C). The patient was considered unable to tolerate a thyroidectomy, so instead RFA was performed. We used Valleylab Cool-Tip™ RFA System (Boulder, CO, USA), including monopolar electrode, radiofrequency generator and cool-tip pump. The nodule was ablated with fixed electrode technique (FET) (Fig. 1D). After sterilization and local anesthesia with 2% lidocaine, the ablation electrode was inserted into the nodule under ultrasound guidance. The ablation was started with 5 W and increased to 28 W gradually in a 1 cm active tip. It was terminated when the RFA-induced transient hyperechoic zone covered the entire carcinoma and the needle passage was also ablated when the ablation electrode was retracted. It took 7 minutes to complete the ablation. The patient was well informed before RFA was performed.
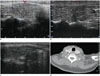
Regular follow-up neck ultrasound showed no signs of recurrence or metastases after 1, 3, 15, and 41 months; and the patient had no complications of RFA (Fig. 2). During the follow-up, the size of ablated lesion was reduced and the echogenicity was decreased; in addition, the TT4 and TT3 were 73.20 nmol/L (55.47–161.25 nmol/L) and 0.94 nmol/L (1.02–2.96 nmol/L), respectively; FT4 and FT3 were 14.02 pmol/L (10.45–24.38 pmol/L) and 3.76 pmol/L (2.77–6.31 pmol/L), respectively. TSH and TPO-Ab were 0.97 mIU/L (0.380–4.340 mIU/L) and 670.70 IU/mL (0–100 IU/mL); respectively.
This case report was approved by the Institutional Review Board.
PTC is the most common subtype of thyroid cancer, comprising > 80% malignant thyroid neoplasms. The 10-year survival rate for PTC is 93% (4). Although the prognosis is excellent, the guidelines of the National Comprehensive Cancer Network for thyroid carcinoma and the European Thyroid Association (ETA) both recommend a total thyroidectomy or lobectomy plus isthmusectomy as the standard treatment for PTC (56).
A non-surgical or minimally invasive treatment is required in thyroid cancer patients who cannot undergo surgery due to severe disease, such as hypertrophic cardiomyopathy. As minimally invasive therapy, RFA induces irreversible damage to tumor tissue with heat generated by high-frequency alternating electric current (7). RFA is an established substitute for surgery for solid cancers such as hepatic cancer (3). RFA is also used to treat benign thyroid nodules and metastatic or recurrent well-differentiated thyroid cancer with difficult reoperations (89). The residual volume after RFA is reduced significantly in benign thyroid nodules and metastatic well-differentiated thyroid carcinoma (89). However, whether RFA is a promising therapeutic option for papillary thyroid microcarcinoma (PTMC), which defined as thyroid cancer ≤ 10 mm in diameter, is unclear.
Baek et al. (10) proposed the moving shot technique (MST) for ablation of thyroid nodules by moving the electrode during the procedure. Compared with FET, MST is more suitable for thyroid gland since it can minimize the risk of heat damage to surrounding critical structures such as recurrent laryngeal nerve, blood vessels and esophagus (11). However, at the time, MST was not in use for thyroid nodules in our hospital, and may have been more suitable for this patient.
Papillary thyroid microcarcinoma rarely progress rapidly so the therapeutic intervention remains controversial. Some surgeons recommend active observation as an appropriate strategy instead of immediate surgery. However, the necessity of at least lobectomy for unifocal PTMC is more accepted. ETA and the American Association of Clinical Endocrinologists recommend partial thyroidectomy and lobectomy plus isthmusectomy for PTMC without neck lymph nodes involvement respectively (512). In our case, a calcified 0.9 × 0.7 × 0.5 cm nodule without enlarged lymph nodes was detected and core needle biopsy confirmed papillary thyroid cancer, so surgery was necessary for the patient. Although surgery is the main therapy for PTMC, our case demonstrates that RFA can be a substitute in patients who cannot tolerate surgery. However, neck lymph nodes metastases are quite common for thyroid cancer and central node involvement reportedly exist in 64% of PTMC cases (13). Therefore, for operable thyroid cancer, RFA should be avoided because of undetectable lymph node metastases and patients undergoing RFA as an alternative to surgery should be followed closely. Signs of recurrence or metastases have not been observed on periodical follow-up neck ultrasound in our patient. However, further observation and clinical trials are needed to compare the value of RFA with surgery. RFA may be a reliable therapeutic candidate even for low-risk PTC patients without contraindication of surgery.
In conclusion, to our knowledge, this is the first reported case of PTC with hypertrophic cardiomyopathy treated with RFA. Despite thyroidectomy as prior therapy in our case, RFA is an alternative when surgery is not feasible. However, further long-term observation is needed to confirm the value of RFA in the treatment of thyroid cancer.
Figures and Tables
Fig. 1
Ultrasound image of thyroid before RFA.
A. Transverse ultrasound image. B. Longitudinal ultrasound image. Solid nodule (0.9 × 0.7 × 0.5 cm) with marked hypoechogenicty, microcalcification, and spiculated margin in right thyroid gland. No enlarged lymph nodes were detected. C. Pathological images of thyroid nodule acquired through core needle biopsy (18 G needle). Classical papillary growth pattern can be observed in image (hematoxylin and eosin stain, magnification × 20). D. Malignant nodule was ablated by ultrasound-guided RFA. Longitudinal ultrasound image showing placement of electrode at target nodule with 1 cm active tip. RFA-induced transient hyperechoic zone covered entire nodule and needle passage was also ablated when ablation electrode was retracted. RFA = radiofrequency ablation
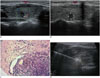
Fig. 2
Follow-up image of thyroid.
A. Longitudinal ultrasound image at 1 month after RFA revealed 1.1 × 0.8 cm ablated mass with slight hyperechogenicity. No recurrent or metastases sign was detected. B. Longitudinal ultrasound image at 3 months after RFA revealed 0.9 × 0.5 cm ablated mass with mix-echogenicity; and no suspicious malignant feature was detected. C. Longitudinal ultrasound image at 15 months after RFA revealed 0.9 × 0.6 cm ablated mass with mix-echogenicity; and no suspicious malignant feature was detected. D. Contrast-enhanced computed tomography image at 41 months after RFA. Low density lesion without contrast-enhance was detected in right lobe, no nodule or enlarged lymph nodes were detected. RFA = radiofrequency ablation
References
1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002; 287:1308–1320.
2. Vives M, Roscoe A. Hypertrophic cardiomyopathy: implications for anesthesia. Minerva Anestesiol. 2014; 80:1310–1319.
3. Rhim H, Lim HK. Radiofrequency ablation of hepatocellular carcinoma: pros and cons. Gut Liver. 2010; 4:Suppl 1. S113–S118.
4. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998; 83:2638–2648.
5. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006; 154:787–803.
6. Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010; 8:1228–1274.
7. Shin JH, Baek JH, Ha EJ, Lee JH. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012; 2012:919650.
8. Bernardi S, Dobrinja C, Fabris B, Bazzocchi G, Sabato N, Ulcigrai V, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014; 2014:934595.
9. Lee SJ, Jung SL, Kim BS, Ahn KJ, Choi HS, Lim DJ, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol. 2014; 15:817–826.
10. Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010; 194:1137–1142.
11. Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014; 15:836–843.
12. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010; 16:Suppl 1. 1–43.
13. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download