Abstract
Objective
By using a functional magnetic resonance imaging (fMRI) technique we assessed brain activation patterns while subjects were viewing the living environments representing natural and urban scenery.
Materials and Methods
A total of 28 healthy right-handed subjects underwent an fMRI on a 3.0 Tesla MRI scanner. The stimulation paradigm consisted of three times the rest condition and two times the activation condition, each of which lasted for 30 and 120 seconds, respectively. During the activation period, each subject viewed natural and urban scenery, respectively.
Results
The predominant brain activation areas observed following exposure to natural scenic views in contrast with urban views included the superior and middle frontal gyri, superior parietal gyrus, precuneus, basal ganglia, superior occipital gyrus, anterior cingulate gyrus, superior temporal gyrus, and insula. On the other hand, the predominant brain activation areas following exposure to urban scenic views in contrast with natural scenes included the middle and inferior occipital gyri, parahippocampal gyrus, hippocampus, amygdala, anterior temporal pole, and inferior frontal gyrus.
Conclusion
Our findings support the idea that the differential functional neuroanatomies for each scenic view are presumably related with subjects' emotional responses to the natural and urban environment, and thus the differential functional neuroanatomy can be utilized as a neural index for the evaluation of friendliness in ecological housing.
Human beings have an instinct to maintain a good quality of life in terms of food, clothing, and living environment. Recently, people are more than ever interested in constructing ecological architecture, natural parks, and green fields to get cheerful and eco-friendly surroundings in urban centers (1, 2). Since the eco-friendly factors contribute much to improve our quality of life, people prefer the comfortable living environments free of physiological fatigue (2, 3). Kaplan and Kaplan (3) asserted that the beauty of nature enhances the interest and concentration, and also reduces the fatigue and stress in our daily life.
Researches on various fields are going on exploring the impacts of the natural and urban environments through various psychological and physiological methodologies in combination with statistical verifications (4-8). These findings suggested that natural environments not only lead to psychological stability such as a calm mood, but also to help in reducing stress. When people look at the natural scenic views, physiological indices such as heart rate, blood pressure, and so on, tend to normalize (4-6). Therefore, living in a nature-friendly environment acts as naturopathy and is a primer for crime prevention and improvements in self-control (7, 8).
The conventional physiological measurements for the evaluation of biocompatibility include the galvanic skin response, heart rate, blood pressure, and electromyogram. Since the 1980s, the electroencephalography (EEG) has been used to access the electrical activity of the localized brain cortex associated with natural and urban environments (9, 10). Ulrich (9) reported that subjects shown urban and scenes produced more alpha (relaxation) activity when viewing nature scenes. High alpha amplitudes are associated with lower levels of physiological arousal as well as feelings of wakeful relaxation. However, the authors did not report specific brain areas associated with natural and urban scenic viewing. Although these studies have provided information regarding the impacts of the natural and urban environments through psychological and physiological methodology, the neural mechanisms associated with the brain activation with natural and urban environmental viewing have not been identified. Recent advances in the neuroimaging techniques such as functional magnetic resonance imaging (fMRI) (11), which makes use of the blood-oxygenation-level-dependent (BOLD) signals from the brain, enable the identification of the neural centers related with the brain function. An fMRI is based on an MRI technique made sensitive to changes in the state of oxygenation of hemoglobin. This non-invasive technique allows us to examine neural mechanisms underlying mental activity like perception or cognition, as well as emotions such as joy, happiness, anger, or sorrow (12-15). The central nervous system plays an important role in inducing emotion via the surrounding environment. Therefore, neuro-scientific evaluation for the surrounding environment in the human life is very important.
In this study, we utilized the BOLD-based fMRI technique to identify the brain centers associated with natural and urban scenic viewing in humans and compare the different activation patterns. The goal of this study is to determine how the natural environments lead to beneficial influences on emotional status in terms of the neural mechanisms.
The subjects were selected on the basis of age and educational level. A total of 28 volunteers, consisting of 16 males (age range: 25-29 years; mean age: 26.9 ± 1.2 years) and 12 females (age range: 20-38 years; mean age: 27.8 ± 5.5 years), were included in this study. All the subjects were right-handed with no history of neurological or psychiatric illness. The subjects were informed of the procedure and matters that required attention prior to the experiment, and written informed consent was obtained. After completion of the fMRI study, each subject was asked to rate their emotional status while viewing the natural and urban sceneries on a 3-point scale: 1, suffocating; 2, accustomed; 3, comfortable. The study was approved by the Chonnam National University Hospital Institutional Review Board (IRB).
The visual stimuli were presented in a block design fashion. The visual stimulation paradigm consisted of three times rest condition and two times activation condition, each lasting for 30 and 120 seconds, respectively. The natural and urban scenic views were presented for 3 seconds each and repeated two times during the activation condition. The rest conditions were a thin white cross mark in the block background screen. Prior to the fMRI examination, a total of 10 subjects were each requested to pick up 20 natural and urban scenic views from a pool of 300 pictures. Pictures for visual stimulation were collected for every 20 natural and urban environment scenic views related to human habitation from a variety of Web sites on the World Wide Web. Natural scenic views included themes such as natural landscapes, mountains, natural parks, forest, and so on. Urban scenic views included themes such as city landscapes, tall buildings, and so on. The average illuminance levels of the natural and urban scenic views were equivalent. The illuminance levels were measured with a digital illuminance meter (Illuminance Meter, Tektronix, Beaverton, OR). The visual stimuli were generated on a PC and projected via a liquid crystal display (LCD) projector onto a screen located on the head coil in front of the subject's forehead. Subjects viewed the screen through a mirror attached to the head coil.
A functional MRI was performed on a 3.0T Magnetom Trio MR Scanner (Siemens Medical Solutions, Forchheim, Germany) with a bird cage head coil. The functional images were acquired from 25 transverse slices parallel to an AC-PC (anterior commissure to posterior commissure) line using a gradient-echo echo planar pulse sequence with the following parameters: repetition time (TR)/echo time (TE) = 2,000 ms/30 ms, flip angle = 90°, field of view (FOV) = 22×22 cm, matrix size = 64×64, number of excitations (NEX) = 1, and slice thickness = 5 mm, giving a total of 4,125 images. In addition, two phases of dummy scans were supplemented to circumvent unstable fMRI signals, for a total acquisition time of 330 seconds. Also, high-resolution anatomical images, T1-weighted images (TR/TE = 500 ms/8 ms), and T2-weighted images (TR/TE = 3,500 ms/88 ms), were acquired with the following parameters: FOV = 22×22 cm, matrix size = 192×192, NEX = 2, slice thickness = 5 mm, and slice gap = 2 mm.
Functional images were analyzed using the SPM99 software (Statistical Parametric Mapping 99, The Wellcome Department of Cognitive Neurology, University College London, UK). At first, images were realigned within and across the scans to correct for head movement. Next, whole-brain normalization was applied to transform all images according to the Montreal Neurological Institute (MNI) template and each volume was resliced with 2 mm3. Normalized images were then smoothed with a spatial Gaussian filter with an 8 mm full-width-at-half-maximum (FWHM). Next, the activated areas were identified by a multiple regression analysis of the time series of the MRI signal intensities in each voxel. The reported coordinates identify the voxel with peak activity within the cluster of activation. The preprocessed date was analyzed using the standard general linear model (GLM) approach of SPM99 with the boxcar model. To analyze the individual BOLD signal in a voxel with a dimension of 2 × 2 × 2 mm, an independent t-test was performed in the rest and activation conditions. Statistical activation maps were obtained for the contrast of 'natural versus rest' and 'urban versus rest'. This analysis was performed in order to identify brain areas with an increased BOLD signal while viewing the natural and urban scenery in relation to the rest periods.
For the group analysis of natural and urban groups, the differential activation maps, which correspond to the contract of natural versus urban and the urban versus natural, were obtained from the two sample t-test. Significant activation maps for these contrasts were identified by a whole-brain analysis with a statistical threshold of p < 0.05.
The questionnaire results evaluating the subjects' emotional status by self-report, while viewing the natural scenery were as follows: comfortable (93%), accustomed (4%), and suffocating (4%). On the other hand, the results of subjects exposed to the urban scenic views were as follows: comfortable (0%), accustomed (50%), and suffocating (50%).
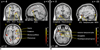
Figure 1 shows the differential brain activation patterns between natural and urban scenic views, which were analyzed by two sample t-test (p < 0.05). The predominant activation areas in natural scenic views in contrast with urban views consist of the superior and middle frontal gyri, superior parietal gyrus, precuneus, basal ganglia, superior occipital gyrus, anterior cingulate gyrus, superior temporal gyrus, and insula. Conversely, the predominant activation areas in urban scenic views in contrast with natural scenic views consist of the middle and inferior occipital gyrus, parahippocampal gyrus, hippocampus, amygdala, anterior temporal pole, and inferior frontal gyrus (Table 1) (Fig. 2).
The aim of this study was to compare the differential brain centers associated with psychological preference between natural and urban scenic views by using the BOLD-based fMRI. Our questionnaire results are consistent with previous studies (6, 16), which suggested that people generally have a preference for natural scenic views over urban views. We assume that such emotional status and behaviors are connected with the activation of the specific brain areas.
In our current study, the predominant brain activation areas following exposure to urban views in contrast to natural scenic views were observed in the middle and inferior occipital gyri, parahippocampal gyrus, hippocampus, amygdala, anterior temporal pole, and inferior frontal gyrus. These results were partly similar to the findings of Lane et al. (12), who had shown the brain activation associated with unpleasant emotion in healthy women using PET (positron emission tomography). They suggested that unpleasant emotion was associated with significant increases in the occipito-temporal cortex, parahippocampal gyrus, hippocampus, and amygdala. In addition, Paradiso et al. (17) suggested that unpleasant stimuli induced the activation of the amygdala and visual cortex in contrast with the pleasant stimuli. In our study, the primary visual cortex and its adjacent areas, which consist of the middle and inferior occipital gyri, showed higher activities in urban views compared to natural views. However, the superior occipital gyrus showed a higher degree of activity in viewing the natural scenic view. The fMRI studies (13, 18, 19) suggested that the primary visual cortex played an important role in visual information perception, and the visual association cortex showed greater enhancement of signal intensities during the processing of emotional pictures. Moreover, increased activity of the primary and association visual cortex was found in the unpleasant/pleasant pictures comparisons (12, 17). It should be noted that the activation of the common areas of the visual cortices evoked by both natural and urban scenic views were eliminated by the statistical analysis with the two sample t-test. Moreover, assuming that viewing the urban scenery leads to an increased emotional arousal compared to natural views, the activation of the middle and inferior occipital gyri noted in our study could be related to a greater arousal level than for mood valence, including pleasant or unpleasant.
We also observed the activation of the parahippocampal gyrus, hippocampus, and amygdala with urban views only. The limbic system, including these areas is related with emotion excitation and affective behavior. The hippocampus and parahippocampal gyrus play the important roles of perception, memory, and recall by visual stimulus (12, 20). In addition, activation in the parahippocampal gyrus and amygdala are associated with recall and re-experiencing of emotional distress as well as distraction for visual stimuli (21). In particular, the parahippocampal gyrus is implicated in the encoding of complex visual pictures (22, 23). The activation of the parahippocampal gyrus noted in our study may reflect encoding of the perceptual aspects related to the visual complexity of urban environments. The amygdala is one of the most important structures in the evaluation of brain activation with negative acquirement and the expressions of anxiety, fear, aversion, and unpleasantness. In our study, the amygdala was activated by urban scenic viewing only. Various studies with humans and animals have suggested that the amygdala responds to aversive stimuli (12, 14, 18, 24). The subjects' self-reported ratings supported that the subjects felt suffocated when viewing the urban scenery compared to than the natural scenery. Siebert et al. (25) demonstrated that normal participants experienced negative emotions from negative visual stimuli, whereas Urbach-Wiethe patients with no amygdala did not experience negative emotions from the same negative stimuli. Such evidence supports that the brain activation in our study may be due to an underlying original nature related to unpleasant emotion while viewing urban scenic views.
The activation of the anterior temporal pole was induced by the urban scenic view only. The anterior temporal pole has reciprocal connections to the amygdala, hippocampus, and prefrontal cortex (26). This area is activated during subjective emotional responses (27), which are associated with negative emotions, including anger (28, 29) and unpleasantness (30). This finding is correlated with self-reported ratings of their unpleasant emotions in viewing the urban scenery over the natural scenic views. Activation of the anterior temporal pole may be related to the appraisal process of negative emotional reactions induced by urban views. The hippocampus, parahippocampal gyrus, amygdale, and anterior temporal pole showed greater activity when exposed to urban scenery, which may be due to the specific role of these areas in processing the unpleasant emotional arousal than the comfortable arousal induced by natural scenic views.
Contrary to the parahippocampal gyrus, hippocampus, and amygdala, the anterior cingulate gyrus, which is also a part of the brain's limbic system, showed greater activity for the natural views. Activation of the anterior cingulate gyrus in our study is typical for mood induction elicited by recall or imagery, as well as during emotional tasks with cognitive demand (31, 32), and is presumably caused by the regulation of tasks with cognitive and affective components when viewing the natural environment. The parietal lobe is often associated with visual and spatial attention. The precuneus is activated when paying attention to a visual, and is also related to episodic memory which generates a visual image during the stimulus (33-35). In our study, the superior parietal gyrus and precuneus were activated while viewing the natural scenery. These results suggest that the subjects showed a tendency to be more concentrated in viewing the urban scenic pictures than the natural scenery, and they did not pay attention to higher visual processing of unpleasant emotion when viewing the stuffy urban scenic pictures.
Another interesting finding in our study is the activation of the insula, which is one of the internal components of the limbic system. The insula showed significant activation in the natural scenic views in contrast with the urban scenery. Some previous neuro-imaging studies (36-38) have demonstrated that the insula is related to a variety of emotional functions. In particular, the insula is preferentially involved in the evaluative, experiential, or expressive aspects of 'internally generated' emotions (37, 39). Also, Bartels and Zeki (40) found significant activation of the insula when viewing the preferred pictures. Conversely, other studies (41, 42) suggested that the insula responded to negative emotional stimuli such as pain and trauma. Thus far, activation of the insula in natural scenic views remains unclear. The basal ganglia include the globus pallidus and caudate nucleus, and its activation is observed in response to happiness-induced recall (38, 43) and pleasant pictures (13, 18, 44). In our study, this area was predominantly activated when viewing the natural scenic pictures only. These findings suggest that the activation of these areas reminds of past experienced memories or recollection by paying greater visual attention to the natural views than the urban scenery.
In our study, the predominant activation areas of the frontal lobe for natural scenic views in contrast with urban scenery included the superior and middle frontal gyri. These areas play an important role in emotional cognitive processes and are thought to be sensitive to the approach-withdrawal emotion specifically (12, 15, 17). The inferior frontal gyrus, another significant area in our study, showed greater brain activity in urban scenic views than natural views. This area may be related with the decision-making and emotional processes associated with urban views. Kross et al. (45) suggested that the rejection sensitivity is associated with a significantly greater level of activity in the inferior frontal gyrus related to self-monitoring. Lane et al. (13) also suggested that the distraction was associated with activation of the inferior frontal gyrus. In particular, the combination of the prefrontal cortex and the limbic system is very important in its brain structure and function. This cortex has a high number of interconnections with the limbic system and thus plays a role in the regulation and expression of emotions and feelings when viewing their favorite or least favorite scenic pictures.
Our current study has some limitations. The human brain perceives a plethora of sensory information of the surrounding environment. However, our study dealt with only the visual stimulation with no regard to other olfactory and auditory stimuli. In addition, we evaluated a subject's emotional status with a self-report while viewing the natural and urban scenery. We did not measure the arousal level of the pictures and the physiological indices reflecting heart rate and blood pressure in this study. Therefore, it is unclear whether the activation areas were related to high or low arousal elicited by natural and urban scenic views.
This study dealt with the evaluation of differential activation patterns of the human brain in response to natural and urban scenic views. The brain activation patterns are presumably associated with personal preference of the scenic views, reflecting a given subject's emotional status and feelings based on psychology. These findings can be utilized as a neural index for the assessment of objective preference of the living environments on human habitation.
Figures and Tables
Fig. 1
Differential brain activation patterns between natural (A) and urban (B) scenic views resulting from two sample t-test. Color-coded pixels on activation maps were scaled to range between cutoff-threshold and highest t-value (p < 0.05).

Fig. 2
Brain activation on sagittal (x), coronal (y), axial (z)-planes demonstrating full details of predominance of natural (A) and urban (B) scenic views, resulting from two sample t test (p < 0.05). SFG = superior frontal gyrus, GLO = globus pallidus, ACG = anterior cingulate gyrus, IFG = inferior frontal gyrus, MOG = middle occipital gyrus
References
1. Bolund P, Hunhammar S. Ecosystem services in urban areas. Ecol Econ. 1999. 29:293–301.
2. Chiesura A. The role of urban parks for the sustainable city. Landsc Urban Plan. 2004. 68:129–138.
3. Kaplan R, Kaplan S. The experience of nature, a psychological perspective. 1989. Cambridge: Cambridge University Press.
4. Banaka WH, Young DW. Community coping skills enhanced by an adventure camp for adult chronic psychiatric patients. Hosp Community Psychiatry. 1985. 36:746–748.
5. Ulrich RS, Simons RF, Losito BD, Fiorito E, Miles MA, Zelson M. Stress recovery during exposure to natural and urban environments. J Environ Psychol. 1991. 11:201–230.
6. Laumann K, Gärling T, Stormark KM. Selective attention and heart rate responses to natural and urban environments. J Environ Psychol. 2003. 23:125–134.
7. Kuo FE, Sullivan WC. Environment and crime in the inner city: does vegetation reduce crime? Environ Behav. 2001. 33:343–367.
8. Taylor AF, Kuo FE, Sullivan WC. Views of nature and self-discipline: evidence from inner city children. J Environ Psychol. 2002. 22:49–63.
9. Ulrich RS. Natural versus urban scenes: some psychophysiological effects. Environ Behav. 1981. 13:523–556.
10. Nakamura R, Fujii E. A comparative study of the characteristics of the electroencephalogram when observing a hedge and a concrete block fence. J Jpn Inst Landscape Arch. 1992. 55:139–144.
11. Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990. 14:68–78.
12. Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997. 35:1437–1444.
13. Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999. 37:989–997.
14. Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000. 20:7752–7759.
15. Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. 2002. 99:4115–4120.
16. Kaplan S, Kaplan R, Wendt JS. Rated preference and complexity for natural and urban visual material. Percept Psychophys. 1972. 12:354–356.
17. Paradiso S, Johnson DL, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999. 156:1618–1629.
18. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997. 154:926–933.
19. Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998. 35:199–210.
20. Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998. 121:1143–1154.
21. Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci. 2004. 1032:254–257.
22. Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003. 100:2157–2162.
23. Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998. 392:598–601.
24. Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997. 94:4119–4124.
25. Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003. 126:2627–2637.
26. Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996. 77:53–77.
27. Lane RD, Fink GR, Chua PM, Dolan RJ. Neural activation during selective attention to subjective emotional response. Neuroreport. 1997. 8:3969–3972.
28. Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, et al. Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry. 1999. 46:466–472.
29. Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999. 46:454–465.
30. Kaplan JT, Freedman J, Iacoboni M. US versus them: political attitudes and party affiliation influence neural response to faces of presidential candidates. Neuropsychologia. 2007. 45:55–64.
31. Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002. 16:331–348.
32. Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Felber S, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage. 2006. 32:854–862.
33. Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye--precuneus activation in memory related imagery. Neuroimage. 1995. 2:195–200.
34. Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002. 15:31–45.
35. Makino Y, Yokosawa K, Takeda Y, Kumada T. Visual search and memory search engage extensive overlapping cerebral cortices: an fMRI study. Neuroimage. 2004. 23:525–533.
36. Jung YC, An SK, Seok JH, Kim JS, Oh SJ, Moon DH, et al. Neural substrates associated with evaluative processing during co-activation of positivity and negativity: a PET investigation. Biol Psychol. 2006. 73:253–261.
37. Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997. 154:918–925.
38. Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000. 3:1049–1056.
39. Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004. 9:258–266.
40. Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000. 11:3829–3834.
41. Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996. 76:571–581.
42. Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002. 13:2023–2026.
43. George MS, Ketter TA, Parekh PI, Herscovitch P, Post RM. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biol Psychiatry. 1996. 40:859–871.
44. Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999. 3:11–21.
45. Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007. 19:945–956.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download