Abstract
We report CT and MR imaging findings for a case of nasal chondromesenchymal hamartoma occurring in a 19-month-old boy. A nasal chondromesenchymal hamartoma is a rare benign pediatric hamartoma that can simulate malignancy. Although rare, knowledge of this entity is essential to avoid potentially harmful therapies.
Anasal chondromesenchymal hamartoma (NCMH) is a rare benign hamartoma of the sinonasal tract, predominantly involving infants under one year of age (1). To date, only 25 cases have been reported in the English literature (1-10). Although the clinical, histopathologic, and CT features have well been described, only limited descriptions of the MR imaging findings have been reported in only a few cases (6-8, 10). We report the CT and MR imaging findings for a case of NCMH occurring in a 19-month-old boy.
A 19-month-old previously healthy boy presented with an 8-month history of watery rhinorrhea and nasal obstruction. The boy was born at full term by transvaginal delivery. Upon examination, the left nasal cavity was obstructed by a large polypoid mass. All other physical findings and laboratory tests were unremarkable.
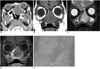
CT scans revealed a 2.7 × 3.5 cm well-defined, expansile mass in the left nasal cavity, extending to the anterior ethmoid sinus. The mass caused pressure remodeling of the adjacent bones without evidence of frank destruction or orbital and intracranial invasion. The results of the precontrast CT scans indicated that the mass was isodense with the cerebral cortex, without evidence of calcification. After contrast enhancement, the lesion demonstrated only minimal enhancement (Fig. 1A). We performed MR imaging to further characterize the internal architecture and found that the signal intensity of the lesion was homogeneously isointense with the cerebral cortex on T1-weighted images (Fig. 1B) and heterogeneously hyperintense on T2-weighted images. The T2-weighted images also showed a peripheral small area of bright signal intensity (Fig. 1C). After contrast enhancement, the majority of the lesion showed a mild diffuse heterogeneous enhancement. The small area of bright signal intensity on the T2-weighted images was seen as a non-enhancing cystic lesion (Fig. 1D).
The patient underwent endoscopic sinus surgery, which revealed a pink-tan soft tissue mass. Resection of the mass was carried out in a piecemeal manner. Microscopically, the lesion consisted of multiple cartilage islands in a myxoid stroma containing spindle cells in a storiform pattern (Fig. 1E). The immunohistochemical staining using a standard immunoperoxidase technique was positive for smooth muscle actin and S-100, and negative for cytokeratin. On the basis of these microscopic and immunohistochemical findings, the diagnosis of the NCMH was made. Upon follow-up MRI examinations obtained one year and four years post-surgery, we found a small lesion in the left ethmoid sinus that had signal characteristics and enhancement pattern which were consistent with the original mass. Although stable, the differentiation between residual tumor and mucosal reaction was difficult. Endoscopic surgery was repeated and the lesion proved to be same histology as the original NCMH. At present, the patient is free of disease for the last 10 months.
An NCMH is a rare benign tumefactive process arising in the nasal cavity and/or paranasal sinuses and occurs predominantly in infants. In 1998, McDermott et al. (1) suggested the term 'nasal chondromesenchymal hamartoma' as a distinct pathologic entity that showed the characteristic histologic features of mixed stromal and chondroid tissue in various proportions, reminiscent of the mesenchymal hamartoma of the chest wall. Before then, an NCMH has been described under the various names, such as chondroid hamartoma, mesenchymoma, and nasal hamartoma (1, 4-7, 10). To date, only 25 cases have been reported in the English literature (1-10). The pathogenesis of NCMH is still unknown. Although originally thought to be developmental or congenital, it seems unlikely because there are documented cases that affect adults with an asymptomatic childhood. It may be that the tumor is caused by an underlying genetic predisposition in combination with the proper stimulation (4). This stimulation could be environmental and may be linked to chronic inflammation or hormones (5).
A review of the 26 cases reported in the literature including the present case indicate that NCMHs occur predominantly in males (19 male and 7 female patients) and infants under one year old (15 patients). The remaining 11 patients, including our patient, ranged between 19 months to 69 years of age (1-5). The clinical manifestations depend on the size and location of the lesion and include respiratory and feeding difficulties, rhinorrhea, epistaxis, visual disturbances, and otitis media. Occasionally, ophthalmologic signs such as ophthalmoplegia, proptosis, ptosis, hypotropia, and enophthalmos can result from the orbital involvement of the tumor (3, 5, 9, 10). An intracranial extension can result in neurologic signs such as hydrocephalus and oculomotor disturbances (5). To date, there have been no reported cases of malignant transformation.
Microscopically, an NCMH is characterized by a variety of mesenchymal components with a focally lobular architecture. The most prominent components are irregular islands of mature and immature hyaline cartilage with occasional binucleated chondrocytes. The islands of cartilage are well demarcated from the surrounding stromal tissues, which have a myxoid background and consist of a relatively bland and compact spindle cell population with variable cellularity (1, 5). To date, no atypical mitotic figures or malignant characteristics have been found. However, reactive bone, small thick-walled vessels, cystic formation, and erythrocyte-filled spaces, have been reported (1, 3). The immunohistochemistry characteristics show positivity to smooth muscle actin, S-100, vimentin, KP-1, and Leu-7, while immunoreactivities for cytokeratin, epithelial membrane antigen, and desmin are negative (5, 6).
Currently, the treatment of choice for an NCMH is complete surgical excision (1, 5, 10). If the lesion is confined to the nasal cavity, it is often amenable to endoscopic surgery. However, the infiltrative nature of the lesion can make it difficult to obtain clean margins (5). Recurrence of an NCMH after surgery has been reported in patients with incomplete resections or microscopic deposits of residual tumor (1, 7). Although radiation therapy and chemotherapy have been applied for lesions that are not completely resected (5), limited clinical experience makes it difficult to conclude how to treat residual or unresectable lesions at this moment.
Diagnostic imaging is useful to evaluate the status of the adjacent structures such as the paranasal sinuses, orbit, and intracranial cavity. Although benign, an NCMH may reveal an aggressive feature with bony erosion, thinning, and displacement, and can raise the suspicion for malignancy. Intracranial extension through the cribriform plate is not infrequent (3, 5, 7, 10). However, frank bony destruction as seen in malignant tumors is not a feature of NCMH (3). Upon imaging, an NCMH is typically seen as a nonencapsulated, poorly defined mass often with cystic components (3, 7). Half of the cases are reported to contain calcifications on CT scans (5). The MR imaging findings of an NCMH have been reported in several patients, mostly in non-radiology journals (2, 6-10). Accordingly, the signal characteristics and enhancement patterns are not specifically described in most cases. In our case, the signal intensity was homogeneously isointense to the cerebral cortex on T1-weighted images and heterogeneously hyperintense on T2-weighted images. These signal intensities were thought to be ascribed to abundant stromal myxoid tissues with relatively low cellularity. Cystic changes can also be well demonstrated as areas of bright signal intensity in relation to the cerebrospinal fluid. In certain cases, the cystic change is so extensive that the tumor can masquerade as a meningoencephalocele (8). In addition, the enhancement patterns can be variable (6, 7, 10). Although our case demonstrated only mild enhancement, there are tumors with prominent vascular proliferation that mimic an angiofibroma histologically (7). Different tissue components and vascularities might cause varied enhancement patterns on imaging.
Upon imaging, differential diagnoses for a pediatric sinonasal mass are lengthy and includes hemangioma, angiofibroma, nasal glioma, inverted papilloma, giant cell reparative granuloma, ossifying fibroma, chondro-osseous respiratory adenomatoid hamartoma, aneurysmal bone cyst, rhabdomyosarcoma, esthesioneuroblastoma, and a chondrosarcoma, with the latter two usually seen only in adolescents (5). Although a case of hypervascular NCMH has been reported, marked enhancement as seen in a hemangioma or angiofibroma is unusual for NCMH. Compared with the cerebral cortex, a nasal glioma usually demonstrates an isointense signal on both the T1- and T2-weighted MR images. Sinonasal inverted papilloma predominantly occurs in adults and is characterized by a convoluted cerebriform pattern on T2-weighted and contrast-enhanced T1-weighted MR images. If the nasal mass contains internal calcifications, a differential diagnosis can be narrowed to ossifying fibroma, chondro-osseous respiratory adenomatoid hamartoma, and chondroid tumor.
In conclusion, we report the CT and MR imaging features of an NCMH, a rare benign hamartoma, generally in pediatric patients. Knowledge of this entity is essential to avoid potentially harmful therapies, since it often simulates malignancy on imaging.
Figures and Tables
Fig. 1
Case of nasal chondromesenchymal hamartoma in 19-month-old boy.
A. Axial postcontrast CT scan shows well-defined, minimally enhancing, expansile mass in left nasal cavity, extending to anterior ethmoid sinus. Thinning of adjacent bones caused by pressure remodeling is observed without evidence of frank destruction. Nasal septum is also displaced to right. Also noted is opacification of left maxillary sinus due to obstruction by mass. On precontrast CT scans, mass was isodense to cerebral cortex without evidence of intralesional calcification (not shown).
B. Coronal T1-weighted MR image demonstrates homogeneously isointense mass, compared with cerebral cortex.
C. Coronal fat-suppressed T2-weighted MR image demonstrates heterogeneously hyperintense mass, in relation to cerebral cortex, containing small area of bright signal intensity peripherally (arrow). Although lesion abuts orbit and anterior cranial base, direct extension of lesion to orbit and intracranial cavity is not evident.
D. Coronal contrast-enhanced fat-suppressed T1-weighted MR image shows mild, diffuse heterogeneous enhancement of lesion. Small area of bright signal intensity seen on T2-weighted image is non-enhancing cystic component (arrow). Note fluid-filled left maxillary sinus on B-D.
E. Photomicrograph shows tumor consisting of multiple chondromyxoid islands (I) in spindle cell stroma (Hematoxylin & Eosin staining, × 100).
References
1. McDermott MB, Ponder TB, Dehner LP. Nasal chondromesenchymal hamartoma: an upper respiratory tract analogue of the chest wall mesenchymal hamartoma. Am J Surg Pathol. 1998. 22:425–433.
2. Alrawi M, McDermott M, Orr D, Russell J. Nasal chondromesenchymal hamartoma presenting in an adolescent. Int J Pediatr Otorhinolaryngol. 2003. 67:669–672.
3. Norman ES, Bergman S, Trupiano JK. Nasal chondromesenchymal hamartoma: report of a case and review of the literature. Pediatr Dev Pathol. 2004. 7:517–520.
4. Ozolek JA, Carrau R, Barnes EL, Hunt JL. Nasal chondromesenchymal hamartoma in older children and adults: series and immunohistochemical analysis. Arch Pathol Lab Med. 2005. 129:1444–1450.
5. Johnson C, Nagaraj U, Esguerra J, Wasdahl D, Wurzbach D. Nasal chondromesenchymal hamartoma: radiographic and histopathologic analysis of a rare pediatric tumor. Pediatr Radiol. 2007. 37:101–104.
6. Kim DW, Low W, Billman G, Wickersham J, Kearns D. Chondroid hamartoma presenting as a neonatal nasal mass. Int J Pediatr Otorhinolaryngol. 1999. 47:253–259.
7. Hsueh C, Hsueh S, Gonzalez-Crussi F, Lee T, Su J. Nasal chondromesenchymal hamartoma in children: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2001. 125:400–403.
8. Kim B, Park SH, Min HS, Rhee JS, Wang KC. Nasal chondromesenchymal hamartoma of infancy clinically mimicking meningoencephalocele. Pediatr Neurosurg. 2004. 40:136–140.
9. Silkiss RZ, Mudvari SS, Shetlar D. Ophthalmologic presentation of nasal chondromesenchymal hamartoma in an infant. Ophthal Plast Reconstr Surg. 2007. 23:243–244.
10. Finitsis S, Giavroglou C, Potsi S, Constantinidis I, Mpaltatzidis A, Rachovitsas D, et al. Nasal chondromesenchymal hamartoma in a child. Cardiovasc Intervent Radiol. 2009. 32:593–597.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download