Abstract
A chondrolipoma is an extremely rare form of a benign mesenchymal tumor containing mature cartilage and fatty tissue. Chondrolipomas may be found in almost any part of the body, particularly in the connective tissue of the breast, head and neck area, as well as in the skeletal muscle. However, to the best of our knowledge, chondrolipomas located in the pelvic cavity have not been reported. In this case report, we describe a case of a chondrolipoma in the pelvis, and show that it has its own characteristic imaging findings, which included the composition of fatty tissue and calcification in most parts, as well as some focal areas of chondroid tissue based on the CT and MR findings.
Achondrolipoma is an extremely rare form of a benign mesenchymal tumor containing mature cartilage and fatty tissue. Only a limited number of chondrolipomas have been reported in the English literature. According to these reports, chondrolipomas may be found almost anywhere in the body, particularly in the connective tissue of the breast (1, 2), head and neck area (3, 4), as well as the skeletal muscle (5, 6). To the best of our knowledge, chondrolipomas have not yet been reported in the pelvic cavity. Herein, we report a case of a chondrolipoma in the pelvis, and describe its CT and MR findings.
A 55-year-old man, presented to our hospital, complained of a large pelvic mass, which was found incidentally during a routine ultrasonography. The patient had no history of abdominal or pelvic discomfort, nor any abdominal or pelvic surgery. In addition, the patient had no relevant medical or family history except for diabetes mellitus. Upon a digital rectal examination, a large mass with a stone-like hardness was palpated at 5-6 cm above the anal verge.
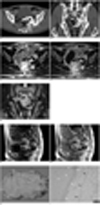
A single-phase helical CT (LightSpeed Ultra; GE Healthcare, Milwaukee, WI), spanning the whole abdominopelvic cavity, was performed. The CT scanning parameters included a beam collimation of 8 × 1.25 mm, a reconstruction interval of 1.25 mm, a pitch of 1.35, a rotation time of 0.8 seconds, a table speed of 13.5 mm/rotation, 120 kV and 160 mAs. Transverse CT images were obtained 70 seconds after the intravenous injection of non-ionic contrast material (120 mL) at a rate of 3 mL/sec (Ultravist 300; Bayer Schering Pharma, Berlin, Germany). Multi-planar reformatted coronal images were reconstructed with the transverse images. The CT images revealed a large, well-defined mass in the presacral space of the pelvic cavity, which seemed to have a long stalk arising from the left gluteus medius muscle (Fig. 1A, B). The stalk traversed along the surface of the left iliac bone, and proceeded through the left sciatic foramen, and ended up connecting with the mass. The CT attenuation generated an easily recognizable mass, which was mainly composed of fatty tissue and calcification, with most of the stalk becoming calcified. The calcification in the mass was located peripherally in a linear and rosary pattern. Some focal areas of intermediate attenuation were identified as a mixture between fatty tissue and calcification, and occupied only a limited portion of the mass. These areas were revealed to correspond to chondroid tissue based on the histologic findings. MR imaging (Horizon 1.5T; GE Healthcare, Milwaukee, WI) was also performed with the use of a TORSO coil. On both the T1-weighted (366/8, echo train length of zero, 5-mm slice thickness, 2-mm gap, 256×192 matrix, 24-cm field of view, 2 signal acquired, and sequence duration of 3-4 minutes) and T2-weighted (4300/84, echo train length of ten, 5-mm slice thickness, 2-mm gap, 512×256 matrix, 24-cm field of view, 2 signal acquired, and sequence duration of 3-4 minutes) MR images, the mass was revealed to mainly consist of the hyperintense and signal void areas that represent fatty tissue and calcification, respectively (Fig. 1C-E). Also seen are the focal areas of intermediate signal intensity, as shown on the CT images. The gadolinium-enhanced T1-weighted MR images obtained 30 seconds, 60 seconds, and 3 minutes after the intravenous injection of contrast material (Gadovist; Bayer Schering Pharma, Berlin, Germany), and the focal areas of the intermediate signal intensity around the calcifications observed at 30 and 60 seconds post-injection, became isointense as the surrounding fat tissue at 3 minutes post-injection. This finding represented a delayed enhancement (Fig. 1F, G). No fat suppression technique was applied on our MR images.
The main mass was resected using a transabdominal approach, whereas the stalk was removed through the sciatic foramen. The surgical specimen consisted of a 13 cm, well-circumscribed, hard mass, with a severely calcified external surface. The cut surface of the resected specimen revealed a mass mixed with fatty tissue and calcification (Fig. 1H). A histopathological examination revealed that the mass was composed of lipoma with chondroid metaplasia (Fig. 1I), as well as extensive calcification within the chondroid tissue. In addition, fibrous tissue was also intermingled within the chondroid tissue. The final histological diagnosis was identified as a chondrolipoma.
The term mesenchymoma was originally defined by Stout (7) in 1948 to describe tumors containing at least two mesenchymal tissues not normally found together. The tumors can be classified as benign or malignant, and most of the benign varieties have been called angiomyolipomas, angiolipomas, chondrolipomas, osteolipomas, and other names depending on the predominant tissue form in the tumor. However, fibrous tissue is found in all mesenchymal tumors, and is not counted as one of the elements (6). Conversely, if a mesenchymal tumor is well-defined or encapsulated and composed predominantly of one type of mesenchymal tissue, along with one or more minor element, the diagnosis should reflect the predominant mesenchymal tissue (6). In our case, the diagnosis of a chondrolipoma was appropriate, since the mass was composed of a sufficiently high proportion of both fatty tissue and extensively calcified chondroid tissue, though it was well-defined and encapsulated.
Two possible explanations for the pathogenesis of cartilage and bone formation in benign mesenchymomas have been proposed (5, 6, 8, 9). The first is that cartilage arises from chondro-osseous metaplasias of adipose tissue, presumably as a result of mechanical stress or trophic disturbance. The close proximity or contact with bone and a large joint, myxoid background, or a lipodystrophy-like change, which are often associated with these tumors, suggest a metaplastic process caused by a trophic disturbance or mechanical stress (8-10). The second is that the cartilage may originate from multipotential cells in the mesenchymoma. A previous immunohistochemical study supported the explanation that the pattern of expression for growth factor-β, latent transforming growth factor-β binding protein-1 transforming, and bone morphogenetic protein might play an important role in the transformation of multipotential cells into the chondrolipoma (11). Considering the proximity of the identified chondrolipoma to the iliac bone of our case study, we agree with the first explanation of chondroid-osseous metaplasia, which states that the mass may be a result of a micro-trauma.
Our mass occupied both the inside and outside of the pelvic cavity; however, we could not clarify whether its primary site was the pelvic cavity or the left buttock. According to previously reported cases, chondrolipomas were mostly found in the connective tissue and skeletal muscle. The only reported case of an abdominal chondrolipoma in a human occurred in the small bowel (8), whereas a chondrolipoma was once documented in the pelvic cavity of a dog (12). Considering these reports, we thought that in our case, a chondrolipoma in the buttock might be extended to the pelvic cavity through the sciatic foramen, though the proportion of the mass in the pelvis was greater than in the buttock.
There are few reports pertaining to the imaging features of chondrolipomas of various organs, with most cases describing their MR features (5, 9, 13, 14). For these reports, chondroid and fatty tissues were described as intermediate and high signal intensity areas on T1-weighted images, respectively. Both chondroid and fatty tissues were identified as having a higher signal intensity area than the surrounding muscle, whereas chondroid tissue was depicted as having a higher signal intensity area than fatty tissue on T2-weighted images. Signal void areas corresponding to calcifications were only found in a small portion of these reported cases. For the CT images, fatty tissue was depicted as a low attenuation area (less than -30 Hounsfield units), whereas chondroid tissue was depicted as an intermediate attenuation area.
In our case, the mass was depicted to be primarily composed of fatty tissue and calcification, which matched the CT attenuation and MR signal intensity. Also, we found that additional focal areas of intermediate attenuation or signal intensity corresponded to chondroid tissue. These findings were different from those described in previously reported cases whereby, in our case, the calcification was much more extensive, and the signal intensity of chondroid tissue on the T2-weighted images was intermediate. Moreover, we found extensive calcification located peripherally in a linear and rosary pattern. A previous report also cited the observation of chondroid tissue in a rosary pattern (9). Despite the very limited number of reported cases, we propose that the rosary pattern of either the chondroid tissue or calcification could be a characteristic finding of chondrolipomas, and in our case, the calcification in a rosary pattern was likely a consequence of the dystrophic change involving the chondroid tissue. Moreover, the signal intensity of chondroid tissue was intermediate on the T2-weighted images, and could be explained by the presence of fibrous tissue within the chondroid tissue (Fig. 1I), which reduced the signal intensity of chondroid tissue below the fatty tissue.
It is interesting that the focal areas of intermediate signal intensity around the calcifications showed delayed enhancement on the gadolinium-enhanced MR images. Orui et al. (9) described the presence of enhancing foci between the fatty tissue and chondroid tissue in a chondrolipoma, without clarifying the histopathological findings. Also, there have been no reports of chondrolipomas with delayed enhancement. In our case, the exact histopathology of the focal areas of delayed enhancement was not substantiated. However, keeping in mind that the location of these focal areas corresponded to chondroid tissue, and that chondroid tissue is usually not enhanced, even on the delayed phase, the delayed enhancement of these focal areas may be attributed due to sufficient fibrous tissue within the chondroid tissue.
Despite the characteristic imaging findings of chondrolipomas, fatty tumors such as lipomas or liposarcomas, which contain chondro-osseous differentiation, should be included in the differential diagnosis (9). The treatment of choice for chondrolipomas is surgical excision (5, 8, 14). Local recurrence is rare, and may represent incomplete removal (5, 8).
Conclusively, we experienced the only reported human case of a chondrolipoma in the pelvic cavity, and found that it had characteristic imaging features consistent with fatty tissue and calcification, as well as the areas corresponding to cartilage.
Figures and Tables
Fig. 1
Chondrolipoma in pelvic cavity in 55-year-old man.
A, B. Transverse (A) and coronal (B) CT images show 8 cm, well-defined mass occupying left side of presacral space of pelvic cavity. Mass seems to have long stalk arising from left gluteus medius muscle. Stalk traverses along surface of left iliac bone, and further passes through left sciatic foramen, and finally connects with mass. Mass is mainly composed of fatty tissue and calcification. Note that calcifications in mass are located peripherally in linear and rosary pattern, with most of stalk becoming calcified. There are some focal areas of intermediate attenuation (arrows) between fat tissue and calcification, which corresponds to chondroid tissue histologically.
C-E. Plain T1-weighted transverse (C), T2-weighted transverse (D), T2-weighted coronal (E) MR images without fat suppression show same mass mainly composed of hyperintense areas and signal void areas on both T1- and T2-weighted images which represent fatty tissue and calcification, respectively. Also seen are focal areas of intermediate signal intensity (arrows), which corresponded to chondroid tissue, as seen on CT images.
F, G. Gadolinium-enhanced T1-weighted sagittal MR images without fat suppression, obtained 30 seconds (F) and 3 minutes (G) after contrast injection, show same fatty mass with extensive calcifications. Note focal areas of intermediate signal intensity around calcifications (arrows) in F become isointense as with surrounding fatty tissue in G. This represents delayed enhancement.
H. Cut surface of resected specimen shows 13 cm, yellowish mass with whitish area in upper portion, which inidcates that this mass is composed of yellowish fatty tissue and whitish calcification.
I. Photomicrograph of resected specimen indicates mature, fatty (black arrows), chondroid (white arrows), and fibrous (white arrowheads) tissues (Hematoxylin & Eosin staining, ×100). These findings are consistent with chondrolipoma.
References
1. Banev SG, Filipovski VA. Chondrolipoma of the breast: case report and a review of literature. Breast. 2006. 15:425–426.
2. Fushimi H, Kotoh K, Nishihara K, Fujinaka H, Takao T. Chondrolipoma of the breast: a case report with cytological and histological examination. Histopathology. 1999. 35:478–479.
3. Hietanen J, Makinen J. Chondrolipoma of the tongue. A case report. Int J Oral Maxillofac Surg. 1997. 26:127–128.
4. Halaas YP, Mra Z, Edelman M. Chondrolipoma of the oropharynx. Ear Nose Throat J. 2001. 80:146–147.
5. Candocia FJ, Barlev DM. Chondrolipoma in the palm of a child: sonographic and MR findings. Clin Imaging. 2004. 28:206–208.
6. Ohtsuka H. Chondrolipoma of the popliteal fossa and Japanese reports. J Dermatol. 2006. 33:202–206.
7. Stout AP. Mesenchymoma, the mixed tumor of mesenchymal derivatives. Ann Surg. 1948. 127:278–290.
8. Jung HR, Park WH, Choi SO, Kwon KY, Lee SS. Intestinal chondrolipoma: uncommon cause of bowel obstruction. J Pediatr Surg. 2007. 42:E21–E23.
9. Orui H, Ishikawa A, Tsuchiya T, Takahara M, Ito M, Ogino T. Chondro-osseous differentiation in fat tissue tumors: magnetic resonance imaging with pathologic correlation. Skeletal Radiol. 2000. 29:459–465.
10. Ito R, Fujiwara M, Takagaki K, Nagasako R. Chondrolipoma of the toe. J Dermatol. 2007. 34:570–572.
11. Nakano M, Arai E, Nakajima Y, Nakamura H, Miyazono K, Hirose T. Immunohistochemical study of chondrolipoma: possible importance of transforming growth factor (TGF)-betas, latent TGF-beta binding protein-1 (LTBP-1), and bone morphogenetic protein (BMP) for chondrogenesis in lipoma. J Dermatol. 2003. 30:189–195.
12. Mutinelli F, Vascellari M, Melchiotti E, Bigolaro M, Bozzato E. Intra-pelvic chondrolipoma in a dog. J Comp Pathol. 2007. 137:160–164.
13. Milchgrub S, McMurry NK, Vuitch F, Dorfman HD. Chondrolipoangioma. A cartilage-containing benign mesenchymoma of soft tissue. Cancer. 1990. 66:2636–2641.
14. Ganzer D, Follak N, Lorenz G. Cartilage and bone containing benign mesenchymoma of the thigh and popliteal fossa. Int Orthop. 2000. 24:115–117.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download