Abstract
We report here on a case of solitary fibrous tumor of the retroperitoneum, and the tumor displayed a predominantly myxoid histology. A 56-year-old man presented with an incidentally detected retroperitoneal mass. On the MR images, the mass was observed as having iso-signal intensity on the T1-weighted images and high signal intensity on the fat-saturated T2-weighted images. The mass showed intense enhancement on the Gd-DTPA enhanced T1-weighted images. At surgery, a well-defined solid mass was found in the left retroperitoneum. The histological diagnosis was made as solitary fibrous tumor with a predominantly myxoid histology.
Solitary fibrous tumor (SFT) is a rare tumor of adults that has a mesenchymal cell origin. It was originally described as a serosa-associated tumor in the pleura, but they are currently known to occur in a variety of extrapleural sites, including the retroperitoneum (1). Although extrapleural SFTs show variable signal intensity depending on the differences in the main components of the tumor, they are usually visualized as a heterogeneous hypointensity on T2-weighted images (2, 3). To the best of our knowledge, only a few cases of myxoid SFT that have shown high signal intensity on T2-weighted images have been reported in the literature (4, 5). In this report, we describe the MRI findings of a SFT arising from the retroperitoneum, and the tumor had a predominantly myxoid histology.
A 56-year-old man presented with an incidentally detected retroperitoneal mass. He had undergone abdominal ultrasonography due to epigastric pain at a local hospital. On the ultrasonogram, a well-defined hypoechoic mass was detected in the left retroperitoneum. No abnormalities were noted on the physical examination or on the routine laboratory studies. His past medical history was unremarkable.
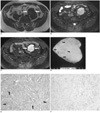
Pelvic MR imaging was performed, for planning surgery, on a 1.5-T unit (Signa; GE Medical Systems, Milwaukee, WI), with using a pelvic phased-array coil. The mass was well circumscribed and slightly lobulated, and it abutted on the left external iliac artery and psoas muscle. The signal intensity on the T1-weighted image was mostly isointense relative to the adjacent muscle (Fig. 1A). The mass was heterogeneously hyperintense and there are several hypointense streaks within the mass on the fatsaturated T2-weighted image (Fig. 1B). The mass showed strong enhancement on the Gd-DTPA enhanced T1-weighted image (Fig. 1C). The preoperative presumptive diagnosis was a retroperitoneal benign mass such as paraganglioma or leiomyoma.
A well-defined solid mass was found in the left retroperitoneum at surgery. The mass abutted on the left external iliac artery and psoas muscle, but there was no evidence of direct invasion. The mass was completely excised. The patient had an uneventful postoperative course and was discharged on the 8th day after his operation.
Gross examination of the mass revealed a yellowish white cut surface with both soft myxoid and rubbery fibrous areas (Fig. 1D). Microscopically, this encapsulated lesion was composed of elongated spindle cells that were dispersed in the heterogeneous stromal matrix with myxoid and collagenous areas (Fig. 1E). There were also multiple small vascular spaces. Any mitotic figures, cellular pleomorphism or nuclear anaplasia were absent. Immunohistochemical analysis showed strong positive marking for CD34 (Fig. 1F), but no immunoreactivity was noted with staining for actin, desmin, S-100, c-kit protein and neurofilament. The final diagnosis was made as SFT with a predominantly myxoid histology.
Extrapleural SFTs have been reported with increasing frequency and they have been described almost everywhere in the body. The retroperitoneum, the deep soft tissues of proximal extremities, abdominal cavity, trunk, head and neck are the most commonly reported extraserosal locations (1). Grossly, SFTs are generally well-circumscribed and slow-growing tumors. Microscopically, they show a wide range of morphological features, from predominantly fibrous lesions that contain large collagenized areas to more cellular and less fibrous neoplasms (1). The histopathological findings are identical regardless of the involved organ, and the imaging features of extrapleural SFTs are similar to those of pleural tumors (6).
Owing to the collagenization and fibrosis, SFTs are usually expected to show low signal intensity on T2-weighted images, which is characteristic of SFTs (3). Kim et al. (7) reported that the signal intensity of SFTs in the head and neck on T2-weighted images is decreased as the collagenous component increased. However, various levels of signal intensities on T2-weighted images have been reported according to the main components of the SFTs (7-9). The high signal intensity of SFTs on T2-weighted images correlated with the myxoid or cystic degeneration, as well as the hypercellularity and the small amount of collagenous matrix (7, 8). Further, malignant fibrous tissue tends to demonstrate high signal intensity on T2-weighted images (9). Although focal myxoid change has been commonly observed and it is well-recognized in SFTs, predominant myxoid variants with 50% or more myxoid stromal change have been described only recently (4, 5). Our case showed predominant myxoid change and this seems to be related to the high signal intensity on T2-weighted images. The linear or curvilinear hypointense lines on MR images that were noted in our case can be attributed to the collagenous stroma (7). Intense enhancement after intravenous gadolinium injection has been reported to be due to the high vascularity of SFTs (2, 7, 8). In fact, the large majority of lesions that have been classified as hemangiopericytoma, which shows an architectural hypervascular pattern, essentially represent SFTs because they show no evidence of pericytic differentiation and instead, they appear to be fibroblastic in nature (1). Thus, hypervascularity and strong enhancement can be the reliable imaging findings of SFTs. Given that SFTs have high vascularity irrespective of the presence of myxoid change, it is unlikely that in our case, the myxoid change would have any effect on the degree of enhancement.
Although most extrapleural SFTs have been reported to be benign histologically, the behavior of extrapleural SFTs is unpredictable and approximately 10-15% of them show recurrent and/or metastatic disease (1). A large tumor size (> 10 cm), increased cellularity with a mitotic index > 4 mitoses per 10 HPF (high power field), and the lack of alternating sclerotic hypocellular areas have all been proposed as the predictors of a poor outcome (10). Yet there is only a poor relationship between morphology and outcome for SFT. With respect to therapy, complete surgical excision with careful long-term follow-up is recommended.
Our tumor in our case should be differentiated from the other solid retroperitoneal masses, including neurogenic tumor such as paraganglioma, leiomyoma, desmoid tumor, inflammatory myofibroblastic tumor and lymphoma. Although the incidence of SFT is low, we suggest that SFT must be included in the differential diagnosis of a retroperitoneal mass, and especially when the mass shows strong enhancement that is irrespective of the signal intensity on the T2-weighted MR image.
In summary, we report here on a case of SFT arising from the retroperitoneum, and the tumor displayed high signal intensity on the T2-weighted MR image, which was attributed to the predominantly myxoid stromal change.
Figures and Tables
Fig. 1
56-year-old man presenting with incidentally detected retroperitoneal mass.
A. Axial T1-weighted MR image shows well-defined, lobulated mass that is isointense to adjacent muscle (arrows).
B. Axial T2-weighted MR image with fat saturation shows that mass is heterogeneously hyperintense to muscle. There are several hypointense streaks (arrows) within mass.
C. Axial Gd-DTPA enhanced T1-weighted MR image demonstrates strong enhancement of mass that abuts on external iliac vessel and psoas muscle (arrows).
D. Sectioned gross pathologic specimen demonstrates yellowish white cut surface with both soft myxoid areas (arrows) and rubbery fibrous areas.
E. Photomicrograph shows elongated spindle cells dispersed in heterogeneous stromal matrix with myxoid areas (arrows) and collagenous areas (original magnification; Hematoxylin-Eosin staining, × 100).
F. Immunohistochemical stain for CD 34. Tumor cells show diffuse strong reactivity for CD 34 (original magnification, × 100).
References
1. Gengler C, Guillou L. Solitary fibrous tumor and haemangiopericytoma: evolution of a concept. Histopathology. 2006. 48:63–74.
2. Vossough A, Torigian DA, Zhang PJ, Siegelman ES, Banner MP. Extrathoracic solitary fibrous tumor of the pelvic peritoneum with central malignant degeneration on CT and MRI. J Magn Reson Imaging. 2005. 22:684–686.
3. Johnson TR, Pedrosa I, Goldsmith J, Dewolf WC, Rofsky NM. Magnetic resonance imaging findings in solitary fibrous tumor of the kidney. J Comput Assist Tomogr. 2005. 29:481–483.
4. de Saint Aubain Somerhausen N, Rubin BP, Fletcher CD. Myxoid solitary fibrous tumor: a study of seven cases with emphasis on differential diagnosis. Mod Pathol. 1999. 12:463–471.
5. Wei YC, Li CF, Sung MT, Chen YT, Ko SF, Eng HL, et al. Primary myxoid solitary fibrous tumor involving the seminal vesicle. Pathol Int. 2006. 56:642–644.
6. Goodlad JR, Fletcher CD. Solitary fibrous tumor arising at unusual sites: analysis of a series. Histopathology. 1991. 19:515–522.
7. Kim HJ, Lee HK, Seo JJ, Kim HJ, Shin JH, Jeong AK, et al. MR imaging of solitary fibrous tumors in the head and neck. Korean J Radiol. 2005. 6:136–142.
8. Chun HJ, Byun JY, Jung SE, Kim KH, Shinn KS. Benign solitary fibrous tumor of the pre-sacral space: MRI findings. Br J Radiol. 1998. 71:677–679.
9. Nagase T, Adachi I, Yamada T, Murakami N, Morita K, Yoshino Y, et al. Solitary fibrous tumor in the pelvic cavity with hypoglycemia: report of a case. Surg Today. 2005. 35:181–184.
10. Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002. 94:1057–1068.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download