Abstract
Objective
To compare the usefulness of magnetic resonance cholangiopancreatography (MRCP) and MR angiography (MRA) in differentiating malignant from benign intraductal papillary mucinous tumors of the pancreas (IPMTs), and to determine the findings which suggest malignancy.
Materials and Methods
During a 6-year period, 46 patients with IPMT underwent MRCP. Morphologically, tumor type was classified as main duct, branch duct, or combined. The diameter of the main pancreatic duct (MPD), the extent of the dilated MPD, and the location and size of the cystic lesion, septum, and communicating channel were assessed. For all types of IPMTs, enhanced mural nodules and portal vein narrowing were evaluated at MRA.
Results
Combined-type IPMTs were more frequently malignant (78%) than benign (42%) (p < 0.05). Compared with benign lesions, malignant lesions were larger, and the caliber of the communicating channel was also larger (p < 0.05). Their dilated MPD was more extensive and of greater diameter (p < 0.05), and the presence of mural nodules was more frequent (p < 0.001).
Intraductal papillary mucinous tumor (IPMT) of the pancreas is a recently recognized pancreatic cystic neoplasm, characterized by diffuse dilatation of the main pancreatic duct (MPD) or branch ducts and the oozing of a variable amount of mucin from the ampulla of Vater (1, 2). IPMTs have been classified as adenoma, borderline, and carcinoma, the last-mentioned category including both in-situ and invasive varieties (3).
Because of the potential of IPMTs for malignant growth, radical surgical resection is mandatory. It has also been reported, however, that these tumors progress slowly and have a good prognosis after resection; thus, some investigators have claimed that compared with malignant IPMTs, those that are benign do not require immediate surgery or very aggressive surgical procedures (4-6). In addition, the surgical strategy employed may be affected by the grade of malignancy (7). In view of the above, if a course of treatment is to be planned, differentiation between malignant and benign IPMTs is essential.
Several recent reports have described the superiority of magnetic resonance cholangiopancreatography (MRCP) to endoscopic retrograde cholangiopancreatography (ERCP) and CT in the evaluation of IPMTs (8-11). However, only one article has assessed the usefulness of MRCP in differentiating malignant from benign IPMT (12). We have found from experience that careful analysis of the source images obtained at contrast-enhanced MR angiography (MRA) can help differentiate malignant from benign IPMTs, But further assessment of this modality is required.
The aim of this study, then, was to compare the usefulness of MRCP and MRA in differentiating malignant from benign intraductal papillary mucinous tumors of the pancreas, and to determine the findings which suggest malignancy.
Between June 1996 and June 2002, IPMT of the pancreas was diagnosed in 64 of our patients on the basis of pathologic examination of surgical specimens. Of these 64, 46 [M:F = 32:14; mean age, 61 (range, 40-77) years] who underwent MRCP and MRA during the month preceding surgery were included in this study. At our hospital, surgical planning requires that most patients in whom IPMT of the pancreas is suspected undergo MRCP and MRA rather than dynamic MR imaging for evaluation of the extent of the disease and the relationship between affected parts and surrounding vessels and organs. These procedures showed that in our 46 patients, the pathologic diagnoses were benign IPMT in 19 patients, borderline IPMT in eight, and malignant IPMT in 19. A borderline lesion was defined as a tumor that was not overtly malignant but had some foci of severe cellular atypia, indicating that it should be treated as malignant. Thus, an IPMT considered at imaging to be 'borderline' was regarded as malignant.
For MRCP examinations, a 1.5-T MR system (Magnetom Vision; Siemens, Erlangen, Germany) was used. Two MRCP techniques were applied: single-slab rapid acquisition with relaxation enhancement (RARE) and multislice half-Fourier acquisition single-shot turbo spin-echo (HASTE). The number of thick-slab acquisitions per patient ranged from three to ten (mean, six). Multislice HASTE images were then obtained in the coronal and oblique planes. Typically, in order to simulate a right anterior oblique projection on direct cholangiography, the oblique plane was at an angle of 20-35° to the coronal plane. The imaging parameters for the single-shot RARE and multislice HASTE sequences have been described in detail elsewhere (13).
For MRA, peak arterial enhancement time was calculated using a test bolus method and 1 mL of gadopentetate dimeglumine (Gd-DTPA). Three-dimensional (3-D) MRA was performed in the coronal plane using two different sequences involving fast imaging with steady precession, the details of which are identical to those previously reported at our hospital (14). Thirty mL (0.2 mmol/kg) of Gd-DTPA were administrated with an automatic power injector (MRS-50; Nemoto, Tokyo, Japan, or Spectris; Medrad, Pittsburgh, Penn., U.S.A.) at a rate of 4 mL/sec, followed by 10 mL of saline flush. The arterial phase was automatically subtracted from precontrast images. In order to fit the portal venous and hepatic venous phases, these were set with a post-arterial phase inter-scan delay of 20 seconds.
The MRCP and MRA images obtained were reviewed retrospectively by two radiologists (K.W.K, S.W.P.) who were aware that the patients had IPMT of the pancreas but had no information regarding the histologic type. All MRCP and MRA sequences in each patient were reviewed, but no attempt was made to evaluate separately the accuracy of individual acquisitions. In cases of interobserver disagreement, final decisions were reached by consensus.
Firstly, tumors were classified as main duct type, branch duct type, or combined type (7). The first of these was diagnosed when dilation of the main pancreatic duct (MPD) had increased its diameter to more than 5 mm. The presence of one or multiple cystic lesions in the pancreas, without dilatation of the MPD, indicated that a branch duct-type tumor was present, and the combined type was diagnosed when the pancreas contained one or more cystic lesions and the diameter of the dilated MPD was more than 5 mm.
Secondly, the reviewers were asked to record the maximum diameter of the MPD and the extent of MPD dilatation; the latter was classified as '< 25%', '25-50%', or '> 50%'. In addition, branch duct-type and combined-type lesions were evaluated in terms of their structure (multilocular or unilocular) and location (whether uncinate process and/or head, or body and/or tail), and the maximal diameter of the entire cystic lesion and the diameter of the largest locule were also determined. Furthermore, decisions were made (subjectively, with regard to the first point) as to whether the lesion had an irregular thick or smooth thin septum, whether there was communication between the lesion and the MPD, and if so, the caliber of the connecting channel.
Lastly, for all types of IPMTs, source and MIP images obtained at MRA were used to determine the presence and largest dimension of enhanced mural nodules, and whether narrowing of the portal vein had occurred.
For each MR finding, we determined the statistical difference between benign and malignant IPMTs by using the chi-square test for qualitative variables and the unpaired Student's t test for numeric values. A p value of less than 0.05 indicated statistical significance.
The MRCP findings for intraductal papillary mucinous tumors are summarized in Table 1. Among the 19 patients with benign IPMT, the main duct type occurred in two (11%) (Fig. 1), the branch duct type in nine (47%) (Fig. 2), and the combined type in eight (42%) (Fig. 3). For the 27 patients with malignant IPMT, the corresponding figures were three (11%), three (11%) (Fig. 4), and 21 (78%) (Fig. 5). The combind type was noticeably more common among malignant tumors (p < 0.05).
Dilatation affected more than 50% of the whole MPD in 59% of malignant IPMTs (16/27) and 26% of benign IPMTs (5/19). The caliber of the MPD ranged from 2 to 14 (6.8 ± 4.2) mm in benign IPMTs, and 4 to 43 (13.3 ± 9.0) mm in malignant IPMTs. The extent of MPD dilatation and the caliber of the MPD were significantly greater in malignant tumors than benign (p < 0.05).
Among branch duct-type and combined-type benign IPMTs, 12 branch duct lesions (71%)were in the uncinate process and/or head, and five were in the body and/or tail. Among these types of malignant IPMTs, 19 lesions (79%) were found in the uncinate process and/or head, and five in the body and/or tail. There were no statistically significant differences in lesion location between benign and malignant IPMTs (p = 0.55).
For branch duct-type and combined-type tumors, the size of the entire cystic lesion was 38.4 ± 17.7 mm if benign, and 55.1 ± 27.2 mm if malignant. The difference was statistically significant (p < 0.05). The average size of the largest locule, for benign and malignant nodules, respectively, was 19.1 ± 9.0 mm and 21.2 ± 10.5 mm. Between benign and malignant IPMTs, there was no statistically significant difference in the size of the largest locule, the structure of the cystic lesions (unilocular or multilocular) (p = 0.08), or the presence or absence of communication between the cystic lesion and the MPD (p = 0.08). In malignant IPMTs, however, the caliber of the communicating tract was significantly larger than in benign IPMTs (p < 0.05).
Mural nodules were observed in 85% (23/27) of malignant IPMTs (Fig. 6), but in only 21% (4/19) of benign IPMTs (p < 0.001). With regard to the presence or absence of portal vein narrowing, however, the difference was not statistically significant (p = 1.6). Only one of 15 benign multilocular branch duct-type lesions had a thick irregular septum, but this was present in 13 of 23 malignant multilocular lesions (57%) (p < 0.001). The mean diameter of mural nodules was 16 ± 11 mm in malignant IPMTs and 6 ± 2 mm in benign IPMTs, representing a statistically significant difference (p < 0.001) (Fig. 7).
Only one published report has described the use of MRCP in the differential diagnosis of IPMT: Irie et al. (12) stated that the size and type of an IPMT, the diameter of a dilated main pancreatic duct, and the presence and size of mural nodules were related to malignancy. Sugiyama and Atomi (4) reported that compared with branch duct-type tumors, main duct and combined-type tumors were more frequently invasive carcinomas, and in our study, combined-type tumors were more often malignant than benign. For main duct types, however, the frequencies with which malignant and benign IPMTs occurred were not significantly different, a finding which can probably be attributed to the small number of main duct-type tumors included in our study group.
Diffuse main pancreatic duct dilatation of greater than 15 mm accompanying main duct-type tumors, or main pancreatic duct dilatation accompanying branch duct-type tumors, is strongly associated with malignancy (12). Communication between branch duct-type IPMT and the MPD was visualized in most benign and malignant IPMTs, but the diameter of the communicating channel was greater in malignant IPMTs than benign IPMTs.
In our study, there was a statistically significant difference between benign and malignant IPMTs as regards both the presence and size of mural nodules. Four benign tumors, however, contained mural nodules, and the presence of such nodules is, therefore, a strongly suggestive but non-specific sign of malignancy. Yamaguchi et al. (5) reported that a mural nodule larger than 10 mm strongly suggests malignancy, and in our study, the size of mural nodules in all four benign IPMTs was less than 10 mm. However, 30% of malignant IPMTs (7/23) also had mural nodules less than 10 mm in size. Kobayashi et al. (15) noted that ductal wall or thickened septum-like structures were irregular in adenocarcinoma and adenoma, but not in hyperplasia. In our study, a thick irregular septum was almost always present in the multilocular cystic lesions arising in branch duct-type malignant IPMTs.
Irie et al. (12) reported that a cystic diameter of 30 mm or more may suggest malignancy, with considerable overlap between benign and malignant IPMTs. In addition, Yanagisawa et al. (16) noted that the greatest diameter of a lesion was larger in malignant IPMTs (50 mm) than benign IPMTs (30 mm), and proposed a criterion of 'larger than 30 mm' as a finding suggestive of malignancy. In our study, too, the average greatest diameter of malignant IPMTs was more than that of benign IPMTs, but because of considerable overlapping, the criterion of 'larger than 30 mm' seemed difficult to apply.
In evaluating mural nodules, we analyzed 3-D MRA source images rather than conventional dynamic MR images; our reason for this was that because of the thin-slice imaging technique employed, the former offer much better image resolution and more physiologic interpretation for evaluation of the entire pancreas and pancreatic duct. In our study, the presence or absence of portal vein narrowing, determined by means of 3-D MRA, was not a statistically significant determinant of benignancy or malignancy. This result was due, we believe, to the low degree of malignancy in our IPMT patients. We consider, though, that in surgical planning for curative resection, the presence or absence of this narrowing is a crucial factor.
In summary, IPMTs are more commonly the combined type. In these and main duct-type tumors, mural nodules larger than 10 mm and a diffuse and markedly dilated MPD suggest malignancy. In branch-duct type IPMTs, this is suggested by a larger cystic lesion, a thick and irregular septum, and a larger-diameter connective tract leading to the MPD. For the differential diagnosis of benign and malignant IPMTs of the pancreas, the combined use of MRCP and MRA might be useful.
Figures and Tables
Fig. 1
A 60-year-old man with benign main duct-type IPMT of the pancreas. Single-slab MRCP image (TR/TE, infinite/1200) shows diffuse dilatation of the main pancreatic duct (arrows).
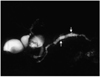
Fig. 2
A 44-year-old man with benign branch duct-type IPMT of the pancreas. Single-slab MRCP image (TR/TE, infinite/1200) depicts a multilocular cystic lesion (arrowheads) in the pancreatic uncinate process and a narrow channel (arrow) which communicates with the pancreatic duct.
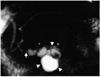
Fig. 3
A 67-year-old man with benign combined duct-type IPMT of the pancreas. Single-slab MRCP image (TR/TE, infinite/1200) reveals a multilocular cystic lesion (arrowheads) in the pancreatic body and diffuse mild dilation of the main pancreatic duct (arrow).

Fig. 4
A 64-year-old woman with malignant branch duct-type IPMT of the pancreas. Single-slab MRCP image (TR/TE, infinite/1200) demonstrates a grapelike cluster of cysts (arrowheads). The main pancreatic duct is not dilated. Another small cystic lesion (arrow), not resected at surgery, is seen in the pancreatic tail.
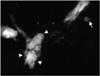
Fig. 5
A 71-year-old man with malignant combined duct-type IPMT of the pancreas. Single-slab MRCP image (TR/TE, infinite/1200) depicts a grapelike cluster of cysts (arrowheads) in the pancreatic head. Marked diffuse dilation of the main pancreatic duct is observed (arrows).
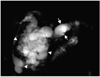
Fig. 6
A 44-year-old man with malignant combined duct-type IPMT of the pancreas. The MRA source image (TR/TE, 4.6/1.8) shows an enhancing mural nodule (arrow) and a thick irregular septum in the multilocular cystic lesion (arrowheads) of the pancreatic head.

References
1. Yamada M, Kozuka S, Yamao K, Nakazawa S, Naitoh Y, Tsukamoto Y. Mucin-producing tumor of the pancreas. Cancer. 1991. 68:159–168.
2. Doi R, Fujimoto K, Wada M, Imamura M. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002. 132:80–85.
3. Kimura W, Sasahira N, Yoshikawa T, Muto T, Makuuchi M. Duct-ectatic type of mucin producing tumor of the pancreas-new concept of pancreatic neoplasia. Hepatogastroenterology. 1996. 43:692–709.
4. Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998. 228:685–691.
5. Yamaguchi K, Ogawa Y, Chijiiwa K, Tanaka M. Mucin-hypersecreting tumors of the pancreas: assessing the grade of malignancy preoperatively. Am J Surg. 1996. 171:427–431.
6. Uehara H, Nakaizumi A, Iishi H, et al. Cytologic examination of pancreatic juice for differential diagnosis of benign and malignant mucin-producing tumors of the pancreas. Cancer. 1994. 74:826–833.
7. Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997. 122:617–625.
8. Sugiyama M, Atomi Y, Hachiya J. Intraductal papillary tumors of the pancreas: evaluation with magnetic resonance cholangiopancreatography. Am J Gastroenterol. 1998. 93:156–159.
9. Koito K, Namieno T, Ichimura T, et al. Mucin-producing pancreatic tumors: comparison of MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Radiology. 1998. 208:231–237.
10. Onaya H, Itai Y, Niitsu M, Chiba T, Michishita N, Saida Y. Ductectatic mucinous cystic neoplasm of the pancreas: evaluation with MR cholangiopancreatography. AJR Am J Roentgenol. 1998. 171:171–177.
11. Fukukura Y, Fujiyoshi F, Sasaki M, et al. HASTE MR cholangiopancreatography in the evaluation of intraductal papillary-mucinous tumors of the pancreas. J Comput Assist Tomogr. 1999. 23:301–305.
12. Irie H, Honda H, Aibe H, et al. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000. 174:1403–1408.
13. Kim TK, Kim BS, Kim JH, et al. Diagnosis of intrahepatic stones: superiority of MR cholangiopancreatography over endoscopic retrograde cholangiopancreatography. AJR Am J Roentgenol. 2002. 179:429–434.
14. Kim JH, Kim TK, Eun HW, et al. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002. 16:676–684.
15. Kobayashi G, Fujita N, Lee S, Kimura K, Watanabe H, Mochizuki F. Correlation between ultrasonographic findings and pathological diagnosis of mucin-producing tumor of the pancreas. Nippon Shokakibyo Gakkai Zasshi. 1990. 87:235–242.
16. Yanagisawa A, Ohashi K, Hori M, et al. Ductectatic-type mucinous cystadenoma and cystadenocarcinoma of the human pancreas: a novel clinicopathological entity. Jpn J Cancer Res. 1993. 84:474–479.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download