Abstract
Purpose
To evaluate the progression of coronary atherosclerotic plaque during follow-up, and its association with cardiovascular risk factors.
Materials and Methods
Fifty-six atherosclerotic patients with plaque were enrolled in this retrospective study. Patient's plaque was detected on repeat 64-slice multidetector CT scans with a mean interval of 25 ± 10 months changes in calcified and non-calcified plaque volumes and cardiovascular risk factors were assessed over time. Absolute and relative changes in plaque volume were compared, and the association between rapid progression and cardiovascular risk factors was determined.
Results
Diameter of the stenosis, length, calcified and non-calcified lesion plaque volumes increased significantly on follow-up CT. Absolute and relative annual changes in plaque volumes were significantly greater in non-calcified plaque (median, 22.7 mm3, 90.4%) than in calcified plaque (median, 0.7 mm3, 0%). Obesity, smoking, hypertension, hypercholesterolemia, and low high-density lipoprotein were significant predictors of progression of non-calcified plaque. Progression of calcified plaque was not associated with any cardiovascular risk factors.
Conclusion
Coronary plaque volume increased significantly on follow-up CT. The rate of progression is related to non-calcified plaque than to calcified plaque. Cardiovascular risk factors are independently associated with the rapid progression of non-calcified plaque volume, but not associated with the progression of calcified plaque.
Coronary heart disease (CHD) is a leading cause of death. Monitoring of coronary atherosclerotic plaque and the effective method for early detection of subclinical CHD have been emphasized (1, 2). Serial assessment of coronary atherosclerotic plaque has contributed to the understanding of the natural history and pathophysiology of CHD (3). Traditionally a coronary angiography has been considered the gold standard method when measuring the extent of atherosclerotic plaque (4). However, this method only depicts the luminal image and is limited in determining plaque composition and explaining the broad spectrum of compensatory coronary arterial remodeling.
Intravascular ultrasound (IVUS) is invasive and therefore not suitable for monitoring progression/regression of coronary plaque (5, 6). On the contrary, coronary CT angiography (CTA) is a non-invasive technique which enables the detection of non-calcified plaque when compared to a non-contrast CT, which detects only calcification (7-9). Thus, the non-invasive repeated CTA could play a role in assessing the changes in coronary atherosclerotic plaque. Defining the relationship between cardiovascular risk factors and changes in plaque burden by a serial CTA would facilitate risk modification in patients with coronary plaque detected by multidetector computed tomography (MDCT). The purpose of this study was to assess the serial changes in coronary plaque volume and stenosis severity during follow-up, and its association with cardiovascular risk factors.
We conducted a retrospective review of the contrast-enhanced coronary CTA patient dataset between April 2007 and March 2011, and identified 168 patients who underwent more than one MDCT examination. Patients had been examined by MDCT due to either a suspected coronary artery disease or a general health examination. With the exception of patients who underwent percutaneous coronary angioplasty (n = 11) and coronary artery bypass graft (n = 5), the MDCT data of the remaining 152 patients were reviewed. The presence of plaque in either of the initial or follow-up CT scans were criteria for inclusion into this study. A total of 56 patients were enrolled in the study. These patients were invited for a follow-up MDCT for clinical indication, which consisted of non angina (10.7%), atypical (25.0%), typical (1.7%) chest pain, or a periodic health examination (62.5%). There was no major cardiovascular event during the follow-up period. This study was approved by the institutional review board of our institute, which waived the need for informed consent due to its retrospective nature.
The pretest probability of CHD in patients based on presenting age, sex, and symptoms was estimated (10). Cardiovascular risk factors for all study participants were obtained through review of medical charts at the time of baseline and follow-up MDCT examinations. Body mass index (BMI) was calculated from measured weight and height, and obesity was defined as BMI > 25 kg/m2. Hypertension was defined by a systolic blood pressure of ≥ 140 mm Hg, diastolic pressure of ≥ 90 mm Hg, or current antihypertensive treatment. Smoking was defined by current or previous daily cigarette use. Diabetes mellitus was defined as a confirmed diagnosis, or by the use of any anti-diabetic medication at entry into the study. Hypercholesterolemia was defined by a total cholesterol ≥ 200 mg/dL or treatment with a lipid-lowering medication- which were defined as on-going statin treatment at entry into the study. A family history of CHD was defined as previous symptomatic CHD treated with medication, or coronary revascularization therapy in a first-degree relative (< 55 years of age for males and < 65 years of age for females). Finally, the 10-year CHD risk based on the Framingham risk score and the modified National Cholesterol Education Program (NCEP) risk category were calculated for each patient (11, 12).
Baseline and follow-up MDCT data were acquired using the Brilliance 64 (Philips Medical Systems, Best, The Netherlands). Non-enhanced imaging was performed to calculate calcium score and volume using 120 kV and 55 mAs with prospective gating. Contrast medium (Iomeron 400, Bracco SPA, Milan, Italy) was injected intravenously at a rate of 4.5 mL/sec for all CTA examinations, followed by a 40 mL saline flush at 4 mL/sec. Scanning was automatically initiated 6 seconds after a threshold of 150 Hounsfield units (HU) was achieved in the region of interest in the descending aorta. CTA was performed with a detector collimation of 64 × 0.625 mm, a 0.4 sec gantry rotation, a pitch of 0.2, an effective tube current of 600-900 mAs with electrocardiography (ECG) modulation, and a tube voltage of 120 kV. Patients received an oral beta-blocker (50-100 mg metoprolol) 1 hour before the examination if they had a rapid heart beat (≥ 70 bpm), assuming no contraindications. Additionally, sublingual nitroglycerin (Nitroglycerin Sublingual Tab. 0.6 mg, Hana, Seoul, Korea) was administered 1 min before image acquisition to dilate the coronary arteries. Reconstructions were performed routinely at the 75% phase of the R-R interval period. Additional cardiac cycles were explored if an adequate image for analysis could not be reconstructed.
MDCT data were transferred to a workstation (Extended Brilliance workspace; Philips Medical Systems, Best, The Netherlands) for post-processing. Oblique multi-planar reformations (MPRs), maximum intensity projection, curved MPRs along the axis of each vessel, two-dimensional map images, and the volume-rendered technique were reconstructed. To calculate the Agatston calcium scores and calcified plaque volume, a semi-automated software identified and defined at least three contiguous pixels (area, 1.03 mm2) with a density of > 130 HU as calcified plaque. Datasets were transferred to an image processing work station (Aquarius, TeraRecon, San Mateo, CA, USA) to evaluate the non-calcified plaque volume on both the baseline and follow-up CTA. After the lesion was identified by two observers by consensus, the analysis was performed by an experienced observer blinded to the patient name and study date (Fig. 1). After automatic contour detection of coronary vessels of ≥ 2 mm in diameter, manual correction was done to trace the leading edges of the luminal and outer border.
In the first step, stenosis diameter was measured using the ratio of the average diameter between the site of maximal luminal narrowing and reference sites. The reference sites were defined as segments without detectable plaque, proximal and distal to, and as close as possible to the respective coronary lesion. In cases in which it was difficult to set both proximal and distal references, the best side of the segment was used as the reference site. In the second step, lesion length was measured from the proximal to the distal shoulder of the plaque. Lastly, the plaque was characterized by color coding based on CT value (HU). Referring to published studies (13-15), the software color codes of the non-calcified coronary plaque were classified into low attenuation plaque (0-50 HU), lipid-rich plaque, intermediate attenuation plaque (51-120 HU), fibrous plaque; and the volume of each component was measured.
The color coding area was manually adjusted to include the full thickness of the vessel wall and to exclude surrounding tissue, such as myocardium, atrium, and the coronary veins. To match the lesions at baseline and follow-up, two curved MPR images of the baseline and follow-up CT were displayed at parallel using workstation, and then were compared side by side to confirm the identical segment based on the adjacent anatomical landmark. The contrast-enhanced coronary artery lumen CT values of the lesion segments at baseline and follow-up CTA were recorded. Non-evaluable segments due to motion artifacts were excluded from analysis. A re-analysis was performed three months after the original analysis to assess the intra-observer agreement.
Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA) software package was used for data collection. Continuous measures were presented as means ± standard deviation or medians with 95% confidence interval (CI). A comparison between baseline and follow-up was performed using the paired t-test for continuous variables and the chi-square test for categorical data. Non-normally distributed continuous variables were compared by the Wilcoxon signed-rank test or Mann-Whitney test. Intra-observer agreement for each measurement was estimated using the intra-class correlation coefficient (ICC). The absolute annual changes in stenosis diameter, lesion length, and plaque volume were assessed by subtracting the values measured at the baseline CT from those measured at the follow-up CT for each plaque, and dividing the difference by the elapsed time between the two measurements. Relative (percentage) annual change was obtained by dividing the absolute annual change by the amount at the baseline scan. At that time, the cases without detectable plaque on both baseline and follow-up CT were considered no change (0%), whereas cases without visible plaque on only baseline CT were excluded from the annual relative change data. Logistic regression analysis was used to determine whether baseline clinical and laboratory variables were predictive of rapid plaque progression. Variables that achieved significance in a univariate analysis were then selected for testing in a stepwise multiple logistic regression analysis. For dichotomous dependent variables, median annual change values for each plaque volume were selected as a cut-off for rapid plaque progression. MedCalc (version 12.2.1; MedCalc Software, Mariakerke, Belgium) was used for all statistical analyses. A p value < 0.05 was considered to indicate statistical significance.
Of the 56 patients included, 44 were male and 12 were female. The mean age was 55 ± 9 years. Clinical characteristics of the 56 patients at baseline are summarized in Table 1. A follow-up CT with a cardiovascular risk profile reacquisition was undertaken a mean of 25 ± 10 months (range, 8-45 months) after the baseline examination. During the follow-up period, only 12 patients were treated with a statin drug. The remaining patients were managed by usual cardiovascular risk factor control, including maintenance of desirable body weight, eating a healthy diet, exercising regularly and not smoking. The changes in cardiovascular risk profiles between baseline and follow-up are listed in Table 2. The Agatston calcium score increased significantly at follow-up. High density lipoprotein (HDL) and low density lipoprotein (LDL) levels decreased significantly at follow-up. Additionally, the Framingham 10 year CHD risk and the NCEP risk categories were significantly upgraded at the follow-up. No significant differences were observed for BMI or total cholesterol and triglyceride levels.
Heart rates during the scan were 62 ± 9 beats/min and 58 ± 8 beats/min in baseline and follow-up. Radiation dose for a single MDCT examination was -12 mSv. Coronary artery lesions were detected in 54 patients on baseline CT. Lesions progressed in two of these patients on follow-up CT. One patient had a coronary artery lesion on only baseline CT. A total of 111 segments with coronary plaques detected on baseline or follow-up CT were included in this analysis, excluding 9 segments (7.5%) with any artifact that might impair plaque assessment. While only one segment was detected on baseline CT, coronary artery lesions were detected in 109 segments, and then developed into two segments on follow-up CT.
The 111 segments consisted of a proximal left anterior descending (LAD, n = 34), mid-LAD (n = 19), proximal right coronary artery (RCA, n = 16), left main (n = 12) and mid-RCA (n = 11), proximal left circumflex (LCX, n = 11), distal LAD (n = 2), distal RCA (n = 2), obtuse marginal branch (n = 2), first diagonal branch (n = 1), and the distal LCX (n = 1). Stenosis severity in all segments evaluated was < 50% on baseline CTA, however 20 segments in 15 patients showed significant stenosis on the follow-up CTA. Eight of the 15 patients underwent invasive coronary angiography, and five underwent revascularization therapy with a coronary stent.
Stenosis diameter, lesion length, and calcified and non-calcified plaque volumes of the coronary lesions increased significantly during the follow-up period (Table 3). Absolute and relative annual changes in diameter stenosis, lesion length, and calcified and non-calcified plaque volumes were 3.3% (95% CI, 2.7-5.3%) and 13.2% (95% CI, 9.7-21.6%), 0.9 mm (95% CI, 0.7-1.3 mm) and 6.0% (95% CI, 4.4-9.0%), 0.7 mm3 (95% CI, 0-2.8 mm3) and 0.0% (95% CI, 0.0-13.0%), and 22.7 mm3 (95% CI, 16.7-32.7 mm3) and 90.4% (95% CI, 34.1-233.0%), respectively. The relative annual change in plaque volume was significantly greater (90.4% vs. 0.0%, p < 0.001) in non-calcified plaque than that in calcified plaque. However, no difference in the relative annual change between lipid-rich and fibrous plaque volumes was observed (120.0% vs. 79.7%, p = 0.487). Twenty one (34%) of 76 segments in 13 patients with non-calcified plaque developed calcification on follow-up CT. Those were excluded from relative annual change analysis, because of zero denominator.
ICC for stenosis diameter, lesion length, and non-calcified plaque volumes between measurements by the same observer were 0.798, 0.837, and 0.875 at the baseline CTA, respectively, and 0.779, 0.839, and 0.846 for the follow-up CTA, respectively. CT values of the coronary artery lumen in the same lesion segments between the baseline and follow-up CT were not significantly different (p = 0.812) on a paired t-test, and almost perfectly concordant (ICC = 0.846) in the intra-class correlation analysis.
The univariate logistic regression analysis of annual plaque change versus cardiovascular risk factors is presented in Table 4. The absolute annual change in non-calcified plaque was significantly associated with obesity, smoking, hypercholesterolemia, triglycerides, HDL, Framingham risk score, and 10-year CHD risk. The relative annual change in non-calcified plaque was significantly associated with obesity, hypertension, smoking, hypercholesterolemia, family history of CHD, HDL, Framingham risk score, 10-year CHD risk, and NCEP risk category. In contrast, absolute and relative annual changes in calcified plaque were not associated with any cardiovascular risk factors.
The cardiovascular risk factors found to be significant in the univariate analysis were included in a multiple logistic regression model (Table 5). The absolute annual change in non-calcified plaque was independently related to obesity, smoking, and HDL. The relative annual change in non-calcified plaque was independently related to obesity, hypertension, smoking, hypercholesterolemia, and HDL.
The major findings of the present study were as follows: First, the coronary atherosclerotic plaques showed progression in plaque volume, luminal stenosis, and lesions length over time. Second, differences were observed in the rate of plaque progression according to plaque composition. The progression of plaque volume was significantly greater in non-calcified plaque compared with that of calcified plaque. Third, the progression of non-calcified plaque was significantly associated with cardiovascular risk factors.
The main challenge for plaque volume measurements is the exact separation between the lumen, plaque, and vessel wall. In particular, delineation of the outer vessel boundary remains problematic, leading to high inter-observer and inter-scan variability (9, 16). However, the reproducibility of plaque volume measurements in recent studies has improved with the introduction of automatic software to quantify plaque. Blackmon et al. (17) reported Pearson's correlation coefficients of 0.781-0.920 according to observer experience. Lee et al. (18) reported that the concordance correlation coefficients for both the intra-observer and inter-observer agreement were 0.90. In the present study, the ICCs for intra-observer agreement were almost perfect (ICC, 0.846-0.875). Another major limitation of plaque quantification is that no standard values of attenuation-based color coding are available for plaque characterization. It is difficult to further classify the non-calcified plaque because of the overlap in CT values of different non-calcified plaque composition (6-8). Moreover, results from a phantom study indicated that intraluminal coronary arterial enhancement has a significant impact on accurate CT densitometry of coronary plaque (19, 20). To overcome this limitation, we compared the intravascular attenuation values of the lesion segments between baseline and follow-up CTA. These results were not significantly different (p = 0.812) on a paired t-test. In addition, non-enhanced images had been used for calcified plaque volume measurement, so we excluded the possible obscuring of the calcium affected by contrast agents.
Several researchers have reported that the rate of serial progression of plaque volume varies. In the present study, the absolute (median, 0.7 mm2) and relative (median, 0.0%) annual changes in calcified plaque were much lower compared with those of previous results (21, 22). Callister et al. (21) reported a 44% median relative annual change in the volumetric score in asymptomatic high-risk individuals. Schmermund et al. (22) reported 32% and 27% of the median relative annual progression of calcium score and area in symptomatic patients, respectively. However, the progression rate depended on the baseline plaque burden. In the present study, which included mainly low-to-intermediate risk patients, 18 (32%) patients on baseline and eight (14%) patients on follow-up had a zero calcium score. In the lesion-based analysis, 53 diseased segments (48%) on baseline CT, and 40 diseased segments (36%) on follow-up CT showed zero calcium. When segments that converted from normal to abnormal calcium were excluded from the analysis, the relative annual change (median, 28.2%; 95% CI, 14.3-36.7%) was similar to previous reports.
In contrast, the progression of non-calcified plaque volume (median, 90.4%; 95% CI, 34.1-233.0%) at follow-up was higher than that reported previously (23, 24). Schmid et al. (23) reported a mean annual progression of 22% (95% CI, 14.7-29.7%) for the volume of non-calcified plaque in the left main and proximal LAD. Inoue et al. (24) reported a non-significant volumetric change (2.1 ± 3.0 vs. 2.3 ± 3.6 mm3, p = 0.2) of low attenuation plaque (< 30 HU) confined to only a 10-mm vascular segment in the non-treated control group. However, we evaluated all segments that showed plaque on CT. Furthermore, since our study population comprised mainly low-to-intermediate risk patients, several baseline volume data approached zero, which resulted in a greater relative annual change.
Although there were limited reports, it seems that non-calcified plaque could progress or regress more rapidly than calcified plaque components (25). We also found more rapid progression (median, 90.4 vs. 0.0%, p < 0.001) of non-calcified than calcified plaque at follow-up. One study found a significant progression in the mean number of cross sections containing non-calcified (p = 0.04) but not calcified (p = 0.2) plaque (26). Non-calcified plaque reflects a feature of early atherosclerotic CHD and is more associated with acute coronary syndrome than calcified plaque (27, 28). Therefore, quantifying the volume change in non-calcified plaque using serial CTA may be a promising shield for acute coronary syndrome. Moreover, this would be most significant if serial assessments were systematically combined with the efficacy of lipid-lowering therapy.
In this present study, the progression of non-calcified plaque was significantly associated with baseline cardiovascular risk factors. In particular, obesity (BMI > 25 kg/m2), smoking history, and HDL level were independent predictors of both absolute and relative annual changes in non-calcified plaque, but calcified plaque was not associated with any risk factors (Table 4). The risk factors correlated with the extent of atherosclerotic plaque are uncertain compared with the relatively well-established relationship between a number of cardiovascular risk factors and clinical event rates (29). Lehman et al. (26) observed that a number of cardiovascular risk factors and smoking were independently associated with plaque progression. Uehara et al. (30) reported that LDL cholesterol level may be an important factor in decreasing non-calcified plaque area. In contrast, Hoffmann et al. (31) found that cardiac risk factors have no significant effect on the plaque growth rate, unlike statin dosage which has a direct effect on the growth rate of non-calcified plaque.
Our findings support a study that found that the rate of change in plaque volume did not differ significantly according to LDL cholesterol level (23). Current guidelines do not recommend CTA screening due to it's radiation hazard, or use of contrast agents due to the expense. Thus, the clinical applications of CTA as a way to monitor for coronary plaque progression remains questionable. Nevertheless our results suggest the importance of risk modification in patients with subclinical CHD.
Some limitations of our study should be addressed. First, this study was retrospective, so the set time period between CT examinations were not explicitly settled. Second, some reports suggest that intensive statin therapy in patients with preexisting coronary disease might induce regression in coronary atheroma burden (32). In our study, only 12 patients were treated with lipid lowering medications during the follow-up and there was no statistically significant relationship between statin therapy and plaque progression. Moreover, most of patients (62.5%) required a follow-up CT scan for periodic health examinations without any optimal medical treatment or risk factor control. Thus these small sample sizes of statin treated or risk controlled groups have limited meaningful analysis of plaque regression.
Third, 2 patients in our study showed an interval increase of calcified plaque volume but a decrease of non-calcified plaque volume. Extensively calcified lesions most likely represent atherosclerosis at later stages of remodeling and may reflect more stable lesions. Our study compared only each quantitative volumetric change and did not consider the effect of calcification of plaque stability. Fourth, the presence of plaque was identified only visually by CT, and MDCT results were not compared to a reference standard such as IVUS. Additionally, the radiation exposure from the repeated MDCT examinations was a major limitation. In our study, the radiation dose for a single MDCT examination was -12 mSv. However recent technical improvements have markedly reduced the radiation dose applied by -45%. This has been accomplished through multiple strategies including usage of low tube voltage in non-obese patients (BMI < 30 or body weight < 90 kg), ECG controlled tube current modulation, prospective ECG triggered scan technique in patients with low heart rates, and a stable sinus rhythm and optimization of scan length (33). These dose reduction technique may increase the acceptability of CT for monitoring the progression/regression of coronary atherosclerotic plaques.
In conclusion, coronary plaque volume increased significantly on follow-up CT, but the rate of progression is greater for non-calcified than for calcified plaque. Additionally, cardiovascular risk factors (obesity, smoking history, and HDL level) were independent predictors of both rapid absolute and relative annual progression of non-calcified plaque volume as assessed by MDCT. No cardiovascular risk factors are associated with the progression of calcified plaque.
Figures and Tables
Fig. 1
Plaque progression in the left anterior descending (LAD) coronary artery at baseline and at the 20 month follow-up in a 43-year-old male patient.
A, B. Agatston calcium score and volume increased from 1.71 to 13.3 and from 5.2 mm2 to 13.3 mm2, respectively.
C, D. Stenosis diameter of the proximal LAD lesion progressed from 6% to 57.9% on the curved multiplanar reformation image.
E, F. Non-calcified plaque volume increased consistently from 4.6 mm2 at baseline to 10.9 mm2 at follow-up.
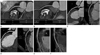
Table 4
Predictors of Rapid Plaque Progression, Univariate Analysis

Note.-Values are odds ratio (95% confidence interval).
*Statistically significant.
BMI = body mass index, CHD = coronary heart disease, HDL = high density lipoprotein, LDL = low density lipoprotein, NCEP = National Cholesterol Education Program, 10 year CHD risk = obtained by Framingham risk score calculator
References
1. Ardehali R, Nasir K, Kolandaivelu A, Budoff MJ, Blumenthal RS. Screening patients for subclinical atherosclerosis with non-contrast cardiac CT. Atherosclerosis. 2007; 192:235–242.
2. Clouse ME. How useful is computed tomography for screening for coronary artery disease? Noninvasive screening for coronary artery disease with computed tomography is useful. Circulation. 2006; 113:125–146. discussion 125-146.
3. Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006; 47:1655–1662.
4. de Feyter PJ, Serruys PW, Davies MJ, Richardson P, Lubsen J, Oliver MF. Quantitative coronary angiography to measure progression and regression of coronary atherosclerosis. Value, limitations, and implications for clinical trials. Circulation. 1991; 84:412–423.
5. Mintz GS, Maehara A. Serial intravascular ultrasound assessment of atherosclerosis progression and regression. State-of-the-art and limitations. Circ J. 2009; 73:1557–1560.
6. Papadopoulou SL, Neefjes LA, Schaap M, Li HL, Capuano E, van der Giessen AG, et al. Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis. 2011; 219:163–170.
7. Cordeiro MA, Lima JA. Atherosclerotic plaque characterization by multidetector row computed tomography angiography. J Am Coll Cardiol. 2006; 47:8 Suppl. C40–C47.
8. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009; 54:49–57.
9. Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006; 47:672–677.
10. Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 2003; 41:159–168.
11. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
12. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004; 110:227–239.
13. Schroeder S, Kuettner A, Leitritz M, Janzen J, Kopp AF, Herdeg C, et al. Reliability of differentiating human coronary plaque morphology using contrast-enhanced multislice spiral computed tomography: a comparison with histology. J Comput Assist Tomogr. 2004; 28:449–454.
14. Soeda T, Uemura S, Morikawa Y, Ishigami K, Okayama S, Hee SJ, et al. Diagnostic accuracy of dual-source computed tomography in the characterization of coronary atherosclerotic plaques: comparison with intravascular optical coherence tomography. Int J Cardiol. 2011; 148:313–318.
15. Bauer RW, Thilo C, Chiaramida SA, Vogl TJ, Costello P, Schoepf UJ. Noncalcified atherosclerotic plaque burden at coronary CT angiography: a better predictor of ischemia at stress myocardial perfusion imaging than calcium score and stenosis severity. AJR Am J Roentgenol. 2009; 193:410–418.
16. Maurovich-Horvat P, Ferencik M, Bamberg F, Hoffmann U. Methods of plaque quantification and characterization by cardiac computed tomography. J Cardiovasc Comput Tomogr. 2009; 3:Suppl 2. S91–S98.
17. Blackmon KN, Streck J, Thilo C, Bastarrika G, Costello P, Schoepf UJ. Reproducibility of automated noncalcified coronary artery plaque burden assessment at coronary CT angiography. J Thorac Imaging. 2009; 24:96–102.
18. Lee MS, Chun EJ, Kim KJ, Kim JA, Vembar M, Choi SI. Reproducibility in the assessment of noncalcified coronary plaque with 256-slice multi-detector CT and automated plaque analysis software. Int J Cardiovasc Imaging. 2010; 26:Suppl 2. 237–244.
19. Cademartiri F, Mollet NR, Runza G, Bruining N, Hamers R, Somers P, et al. Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol. 2005; 15:1426–1431.
20. Horiguchi J, Fujioka C, Kiguchi M, Shen Y, Althoff CE, Yamamoto H, et al. Soft and intermediate plaques in coronary arteries: how accurately can we measure CT attenuation using 64-MDCT? AJR Am J Roentgenol. 2007; 189:981–988.
21. Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998; 339:1972–1978.
22. Schmermund A, Baumgart D, Möhlenkamp S, Kriener P, Pump H, Grönemeyer D, et al. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: an electron-beam CT study. Arterioscler Thromb Vasc Biol. 2001; 21:421–426.
23. Schmid M, Achenbach S, Ropers D, Komatsu S, Ropers U, Daniel WG, et al. Assessment of changes in non-calcified atherosclerotic plaque volume in the left main and left anterior descending coronary arteries over time by 64-slice computed tomography. Am J Cardiol. 2008; 101:579–584.
24. Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, et al. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010; 3:691–698.
25. Priester TC, Litwin SE. Measuring progression of coronary atherosclerosis with computed tomography: searching for clarity among shades of gray. J Cardiovasc Comput Tomogr. 2009; 3:Suppl 2. S81–S90.
26. Lehman SJ, Schlett CL, Bamberg F, Lee H, Donnelly P, Shturman L, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging. 2009; 2:1262–1270.
27. Fernández-Ortiz A, Badimon JJ, Falk E, Fuster V, Meyer B, Mailhac A, et al. Characterization of the relative thrombogenicity of atherosclerotic plaque components: implications for consequences of plaque rupture. J Am Coll Cardiol. 1994; 23:1562–1569.
28. Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, et al. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol. 2008; 28:568–574.
29. Nicholls SJ, Tuzcu EM, Crowe T, Sipahi I, Schoenhagen P, Kapadia S, et al. Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. J Am Coll Cardiol. 2006; 47:1967–1975.
30. Uehara M, Funabashi N, Mikami Y, Shiina Y, Nakamura K, Komuro I. Quantitative effect of atorvastatin on size and content of non-calcified plaques of coronary arteries 1 year after atorvastatin treatment by multislice computed tomography. Int J Cardiol. 2008; 130:269–275.
31. Hoffmann H, Frieler K, Schlattmann P, Hamm B, Dewey M. Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol. 2010; 20:2824–2833.
32. Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006; 295:1556–1565.
33. Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011; 5:198–224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download