1. Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008; 26:5458–5464.
2. Dans AL, Dans LF, Guyatt GH, Richardson S. Evidence-Based Medicine Working Group. Users’ guides to the medical literature: XIV. How to decide on the applicability of clinical trial results to your patient. JAMA. 1998; 279:545–549.
3. Meyer RM. Generalizing the results of cancer clinical trials. J Clin Oncol. 2010; 28:187–189.
4. Arana A, Rivero E, Egberts TC. What do we show and who does so? An analysis of the abstracts presented at the 19th ICPE. Pharmacoepidemiol Drug Saf. 2004; 13:S330–1.
5. Cook C, Sheets C. Clinical equipoise and personal equipoise: two necessary ingredients for reducing bias in manual therapy trials. J Manual Manip Ther. 2011; 19:55–57.
6. García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998; 45:419–425.
7. Chen YC, Wu JC, Chen TJ, Wetter T. Reduced access to database. A publicly available database accelerates academic production. BMJ. 2011; 342:d637.
8. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992; 45:613–619.
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998; 36:8–27.
10. Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996; 53:1022–1031.
11. Park BJ, Sung J, Park K, Seo SW, Kim SH. Studying on diagnosis accuracy for health insurance claims data in Korea. Seoul: Seoul National University;2003.
12. Cho J, Lee WJ, Moon KT, Suh M, Sohn J, Ha KH, Kim C, Shin DC, Jung SH. Medical care utilization during 1 year prior to death in suicides motivated by physical illnesses. J Prev Med Public Health. 2013; 46:147–154.
13. Chung K, Kim K, Jung J, Oh K, Oh Y, Kim S, Kim J, Kim Y. Patterns and determinants of COPD-related healthcare utilization by severity of airway obstruction in Korea. BMC Pulm Med. 2014; 14:27.
14. Jung JY, Kang YA, Park MS, Oh YM, Park EC, Kim HR, Lee SD, Kim SK, Chang J, Kim YS. Chronic obstructive lung disease-related health care utilisation in Korean adults with obstructive lung disease. Int J Tuberc Lung Dis. 2011; 15:824–829.
15. Shin JY, Park MJ, Lee SH, Choi SH, Kim MH, Choi NK, Lee J, Park BJ. Risk of intracranial haemorrhage in antidepressant users with concurrent use of non-steroidal anti-inflammatory drugs: nationwide propensity score matched study. BMJ. 2015; 351:h3517.
16. Lee JY, Jo MW, Yoo WS, Kim HJ, Eun SJ. Evidence of a broken healthcare delivery system in Korea: unnecessary hospital outpatient utilization among patients with a single chronic disease without complications. J Korean Med Sci. 2014; 29:1590–1596.
17. Kim J, Kim K, Kim Y, Yoo KH, Lee CK, Yoon HK, Kim YS, Park YB, Lee JH, Oh YM, et al. The association between inhaled long-acting bronchodilators and less in-hospital care in newly-diagnosed COPD patients. Respir Med. 2014; 108:153–161.
18. Kim SY, Park JH, Kim SG, Woo HK, Park JH, Kim Y, Park EC. Disparities in utilization of high-volume hospitals for cancer surgery: results of a Korean population-based study. Ann Surg Oncol. 2010; 17:2806–2815.
19. Shin S, Jang S, Lee TJ, Kim H. Association between non-adherence to statin and hospitalization for cardiovascular disease and all-cause mortality in a national cohort. Int J Clin Pharmacol Ther. 2014; 52:948–956.
20. Han E, Suh DC, Lee SM, Jang S. The impact of medication adherence on health outcomes for chronic metabolic diseases: a retrospective cohort study. Res Social Adm Pharm. 2014; 10:e87–e98.
21. Ahn SH, Choi NK, Kim YJ, Seong JM, Shin JY, Jung SY, Park BJ. Drug persistency of cholinesterase inhibitors for patients with dementia of Alzheimer type in Korea. Arch Pharm Res. 2015; 38:1255–1262.
22. Cho SK, Sung YK, Choi CB, Bae SC. Impact of comorbidities on TNF inhibitor persistence in rheumatoid arthritis patients: an analysis of Korean National Health Insurance claims data. Rheumatol Int. 2012; 32:3851–3856.
23. Shin DW, Park JH, Park JH, Park EC, Kim SY, Kim SG, Choi JY. Antihypertensive medication adherence in cancer survivors and its affecting factors: results of a Korean population-based study. Support Care Cancer. 2011; 19:211–220.
24. Park JH, Shin Y, Lee SY, Lee SI. Antihypertensive drug medication adherence and its affecting factors in South Korea. Int J Cardiol. 2008; 128:392–398.
25. Rhee CK, Yoon HK, Yoo KH, Kim YS, Lee SW, Park YB, Lee JH, Kim Y, Kim K, Kim J, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD. 2014; 11:163–170.
26. Kim JH, Park JS, Kim KH, Jeong HC, Kim EK, Lee JH. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest. 2013; 143:1018–1024.
27. Kim J, Rhee CK, Yoo KH, Kim YS, Lee SW, Park YB, Lee JH, Oh Y, Lee SD, Kim Y, et al. The health care burden of high grade chronic obstructive pulmonary disease in Korea: analysis of the Korean Health Insurance Review and Assessment Service data. Int J Chron Obstruct Pulmon Dis. 2013; 8:561–568.
28. Kim J, Lee JH, Kim Y, Kim K, Oh YM, Yoo KH, Rhee CK, Yoon HK, Kim YS, Park YB, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013; 13:51.
29. Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiology. 2006; 17:260–267.
30. Kim HA, Kim S, Seo YI, Choi HJ, Seong SC, Song YW, Hunter D, Zhang Y. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology (Oxford). 2008; 47:88–91.
31. Kim SA, Kilgore PE, Lee SY, Nyambat B, Ki M. Trends in pneumonia and influenza-associated hospitalizations in South Korea, 2002-2005. J Health Popul Nutr. 2011; 29:574–582.
32. Kwon GY, Lee H, Gwack J, Lee SW, Ki M, Youn SK. Regional distribution of hepatitis C virus infection in the Republic of Korea, 2007-2011. Gut Liver. 2014; 8:428–432.
33. Lee EJ, Park HM. Trends in laparoscopic surgery for hysterectomy in Korea between 2007 and 2009. J Obstet Gynaecol Res. 2014; 40:1695–1699.
34. Lee JH, Park YS, Choi JS. The epidemiology of appendicitis and appendectomy in South Korea: national registry data. J Epidemiol. 2010; 20:97–105.
35. Lee WJ, Ko Y, Cha ES. Acute pesticide poisoning among children in South Korea: findings from National Health Insurance claims data, 2006-2009. J Trop Pediatr. 2014; 60:4–9.
36. Lee YK, Ha YC, Yoon BH, Koo KH. National trends of hip arthroscopy in Korea. J Korean Med Sci. 2014; 29:277–280.
37. Lee YK, Yoon BH, Nho JH, Kim KC, Ha YC, Koo KH. National trends of surgical treatment for intertrochanteric fractures in Korea. J Korean Med Sci. 2013; 28:1407–1408.
38. Research Data Assistance Center (ResDAC) (US) [Internet]. accessed on 6 November 2016. Available at
http://www.resdac.org/.
39. Chronic Condition Data Warehouse (CCW) (US) [Internet]. accessed on 6 November 2016. Available at
http://www.ccwdata.org/.
40. Ahn YH, Kim ES, Ham OK, Kim SH, Hwang SS, Chun SH, Gwon NY, Choi JY. Factors associated with the overuse or underuse of health care services among medical aid beneficiaries in Korea. J Community Health Nurs. 2011; 28:190–203.
41. Cho GJ, Kim LY, Hong HR, Lee CE, Hong SC, Oh MJ, Kim HJ. Trends in the rates of peripartum hysterectomy and uterine artery embolization. PLoS One. 2013; 8:e60512.
42. Choi HG, Hah JH, Jung YH, Kim DW, Sung MW. Influences of demographic changes and medical insurance status on tonsillectomy and adenoidectomy rates in Korea. Eur Arch Otorhinolaryngol. 2014; 271:2293–2298.
43. Chung W. Psychiatric inpatient expenditures and public health insurance programmes: analysis of a national database covering the entire South Korean population. BMC Health Serv Res. 2010; 10:263.
44. Chung W, Chang HS, Oh SM, Yoon CW. Factors associated with long-stay status in patients with schizophrenia: an analysis of national databases covering the entire Korean population. Int J Soc Psychiatry. 2013; 59:207–216.
45. Han K, Cho M, Chun K. Determinants of health care expenditures and the contribution of associated factors: 16 cities and provinces in Korea, 2003-2010. J Prev Med Public Health. 2013; 46:300–308.
46. Hong JS, Kang HC. Relationship between the use of new or used computed tomography scanners and image retake rates in South Korea. Acta Radiol. 2013; 54:428–434.
47. Kim V, Kim H, Lee K, Chang S, Hur M, Kang J, Kim S, Lee SW, Kim YE. Variation in the numbers of red blood cell units transfused at different medical institution types from 2006 to 2010 in Korea. Ann Lab Med. 2013; 33:331–342.
48. Koh IJ, Kim TK, Chang CB, Cho HJ, In Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin Orthop Relat Res. 2013; 471:1441–1450.
49. Lee HS. The impact of emergency room utilization by depression patients on medical treatment expense in Korea. Osong Public Health Res Perspect. 2013; 4:240–245.
50. Lee K, Kim H, Heo JH, Bae HJ, Koh IS, Chang S. Application of magnetic resonance imaging and magnetic resonance angiography as diagnostic measures for the first attack of suspected cerebrovascular diseases in Korea. Yonsei Med J. 2011; 52:727–733.
51. Park HS, Choi BY, Kwon YD. Rapid increase in the national treatment costs for hepatitis A infections in Korea. Tohoku J Exp Med. 2012; 226:85–93.
52. Kim C, Yoo KH, Rhee CK, Yoon HK, Kim YS, Lee SW, Oh YM, Lee SD, Lee JH, Kim KJ, et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int J Tuberc Lung Dis. 2014; 18:737–743.
53. Kim HA, Koh SH, Lee B, Kim IJ, Seo YI, Song YW, Hunter DJ, Zhang Y. Low rate of total hip replacement as reflected by a low prevalence of hip osteoarthritis in South Korea. Osteoarthritis Cartilage. 2008; 16:1572–1575.
54. Kim YJ, Choi NK, Kim MS, Lee J, Chang Y, Seong JM, Jung SY, Shin JY, Park JE, Park BJ. Evaluation of low-dose aspirin for primary prevention of ischemic stroke among patients with diabetes: a retrospective cohort study. Diabetol Metab Syndr. 2015; 7:8.
55. Lee K, Lee S. Effects of the DRG-based prospective payment system operated by the voluntarily participating providers on the cesarean section rates in Korea. Health Policy. 2007; 81:300–308.
56. Bae G, Park C, Lee H, Han E, Kim DS, Jang S. Effective policy initiatives to constrain lipid-lowering drug expenditure growth in South Korea. BMC Health Serv Res. 2014; 14:100.
57. Heo JH, Suh DC, Kim S, Lee EK. Evaluation of the pilot program on the real-time drug utilization review system in South Korea. Int J Med Inform. 2013; 82:987–995.
58. Kim YJ, Oh Y, Park S, Cho S, Park H. Stratified sampling design based on data mining. Healthc Inform Res. 2013; 19:186–195.
59. Seo HJ, Yoon SJ, Lee SI, Lee KS, Yun YH, Kim EJ, Oh IH. A comparison of the Charlson comorbidity index derived from medical records and claims data from patients undergoing lung cancer surgery in Korea: a population-based investigation. BMC Health Serv Res. 2010; 10:236.
60. Park HK, Yoon SJ, Ahn HS, Ahn LS, Seo HJ, Lee SI, Lee KS. Comparison of risk-adjustment models using administrative or clinical data for outcome prediction in patients after myocardial infarction or coronary bypass surgery in Korea. Int J Clin Pract. 2007; 61:1086–1090.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



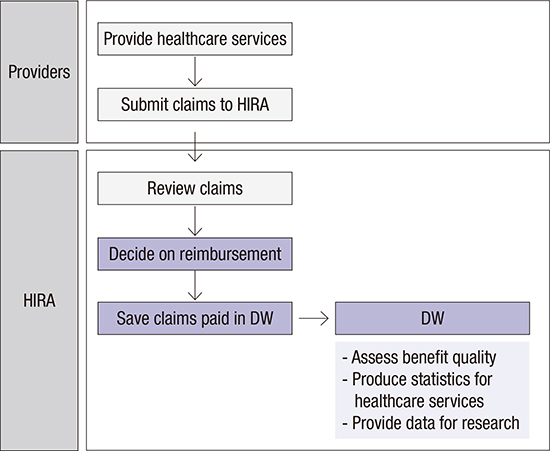
 XML Download
XML Download