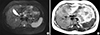
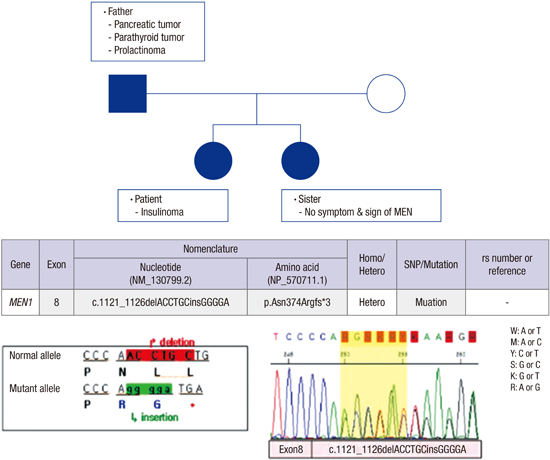
1. LaFranchi S. Hypoglycemia of infancy and childhood. Pediatr Clin North Am. 1987; 34:961–982.
2. Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013; 19:829–837.
3. Iglesias P, Díez JJ. Management of endocrine disease: a clinical update on tumor-induced hypoglycemia. Eur J Endocrinol. 2014; 170:R147–57.
4. Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012; 97:2990–3011.
5. Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009; 94:709–728.
6. Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, Andrews JC, Lloyd RV, Service FJ. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab. 2009; 94:1069–1073.
7. Service FJ, McMahon MM, O’Brien PC, Ballard DJ. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991; 66:711–719.
8. Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, Schneyer U, Goretzki P, Raue F, Dralle H. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf). 2007; 67:613–622.
9. Marini F, Falchetti A, Del Monte F, Carbonell Sala S, Gozzini A, Luzi E, Brandi ML. Multiple endocrine neoplasia type 1. Orphanet J Rare Dis. 2006; 1:38.
10. Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol. 2014; 386:2–15.
11. Trump D, Farren B, Wooding C, Pang JT, Besser GM, Buchanan KD, Edwards CR, Heath DA, Jackson CE, Jansen S, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1). QJM. 1996; 89:653–669.
12. Bassett JH, Forbes SA, Pannett AA, Lloyd SE, Christie PT, Wooding C, Harding B, Besser GM, Edwards CR, Monson JP, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet. 1998; 62:232–244.
13. Turner JJ, Christie PT, Pearce SH, Turnpenny PD, Thakker RV. Diagnostic challenges due to phenocopies: lessons from Multiple Endocrine Neoplasia type1 (MEN1). Hum Mutat. 2010; 31:E1089–101.
14. de Vogelaere K, De Schepper J, Vanhoeij M, De Mey J, Goossens A, Vanbesien J, De Backer A, Delvaux G. Laparoscopic management of insulinoma in a child with multiple endocrine neoplasia type 1. J Laparoendosc Adv Surg Tech A. 2006; 16:335–338.
15. Winston KY, Dawrant J. A rare case of hypoglycaemia due to insulinoma in an adolescent with acutely altered mental status. J Pediatr Endocrinol Metab. 2014; 27:773–776.
16. Panamonta O, Areemit S, Srinakarin J, Puapairoj A. Insulinoma in childhood. J Med Assoc Thai. 2001; 84:136–142.
17. Janem W, Sultan I, Ajlouni F, Deebajeh R, Haddad H, Sughayer MA, Goussous RY. Malignant insulinoma in a child. Pediatr Blood Cancer. 2010; 55:1423–1426.
18. Akerström G, Hellman P. Surgery on neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007; 21:87–109.