1. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989; 321:569–574.
2. Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002; 166:1235–1239.
3. Hayakawa H, Sato A, Toyoshima M, Imokawa S, Taniguchi M. A clinical study of idiopathic eosinophilic pneumonia. Chest. 1994; 105:1462–1466.
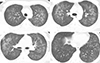
4. Daimon T, Johkoh T, Sumikawa H, Honda O, Fujimoto K, Koga T, Arakawa H, Yanagawa M, Inoue A, Mihara N, et al. Acute eosinophilic pneumonia: thin-section CT findings in 29 patients. Eur J Radiol. 2008; 65:462–467.
5. Rhee CK, Min KH, Yim NY, Lee JE, Lee NR, Chung MP, Jeon K. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013; 41:402–409.
6. Jhun BW, Kim SJ, Kim K, Lee JE. Clinical implications of initial peripheral eosinophilia in acute eosinophilic pneumonia. Respirology. 2014; 19:1059–1065.
7. Lim SY, Suh GY, Jeon K. Acute eosinophilic pneumonia presenting as life-threatening hypoxaemia necessitating extracorporeal membrane oxygenation. Int J Tuberc Lung Dis. 2012; 16:1711–1712.
8. Kawayama T, Fujiki R, Morimitsu Y, Rikimaru T, Aizawa H. Fatal idiopathic acute eosinophilic pneumonia with acute lung injury. Respirology. 2002; 7:373–375.
9. Imokawa S, Sato A, Hayakawa H, Toyoshima M, Taniguchi M, Chida K. Possible involvement of an environmental agent in the development of acute eosinophilic pneumonia. Ann Allergy Asthma Immunol. 1996; 76:419–422.
10. Rom WN, Weiden M, Garcia R, Yie TA, Vathesatogkit P, Tse DB, McGuinness G, Roggli V, Prezant D. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002; 166:797–800.
11. Watanabe K, Fujimura M, Kasahara K, Yasui M, Myou S, Kita T, Watanabe A, Nakao S. Acute eosinophilic pneumonia following cigarette smoking: a case report including cigarette-smoking challenge test. Intern Med. 2002; 41:1016–1020.
12. Uchiyama H, Suda T, Nakamura Y, Shirai M, Gemma H, Shirai T, Toyoshima M, Imokawa S, Yasuda K, Ida M, et al. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008; 133:1174–1180.
13. Kitahara Y, Matsumoto K, Taooka Y, Moritani C, Nakamura K, Ohashi N, Daido K, Arita K. Cigarette smoking-induced acute eosinophilic pneumonia showing tolerance in broncho-alveolar lavage findings. Intern Med. 2003; 42:1016–1021.
14. Shiota Y, Kawai T, Matsumoto H, Hiyama J, Tokuda Y, Marukawa M, Ono T, Mashiba H. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000; 39:830–833.
15. Katoh S, Matsumoto N, Matsumoto K, Fukushima K, Matsukura S. Elevated interleukin-18 levels in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. Allergy. 2004; 59:850–856.
16. Mato N, Bando M, Kusano A, Hirano T, Nakayama M, Uto T, Nakaya T, Yamasawa H, Sugiyama Y. Clinical significance of interleukin 33 (IL-33) in patients with eosinophilic pneumonia. Allergol Int. 2013; 62:45–52.
17. Miyazaki E, Nureki S, Fukami T, Shigenaga T, Ando M, Ito K, Ando H, Sugisaki K, Kumamoto T, Tsuda T. Elevated levels of thymus- and activation-regulated chemokine in bronchoalveolar lavage fluid from patients with eosinophilic pneumonia. Am J Respir Crit Care Med. 2002; 165:1125–1131.
18. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996; 97:1366–1374.
19. Okubo Y, Horie S, Hachiya T, Momose T, Tsukadaira A, Takashi S, Suzuki J, Isobe M, Sekiguchi M. Predominant implication of IL-5 in acute eosinophilic pneumonia: comparison with chronic eosinophilic pneumonia. Int Arch Allergy Immunol. 1998; 116:76–80.
20. Lee JE, Rhee CK, Lim JH, Lee SM, Shim YS, Lee CT, Lee SW. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. 2012; 141:1267–1272.
21. Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ. Clinical implications of correlation between peripheral eosinophil count and serum levels of IL-5 and tryptase in acute eosinophilic pneumonia. Respir Med. 2014; 108:1655–1662.
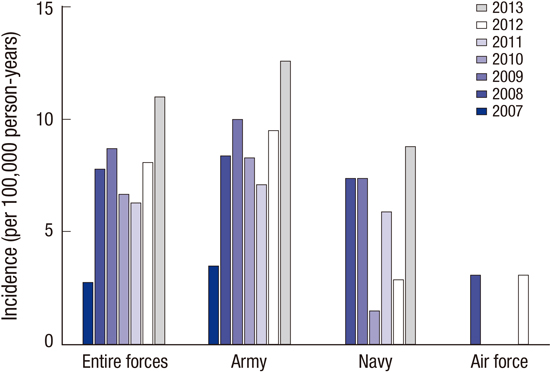
22. Shorr AF, Scoville SL, Cersovsky SB, Shanks GD, Ockenhouse CF, Smoak BL, Carr WW, Petruccelli BP. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004; 292:2997–3005.
23. Matsuno O, Takenaka R, Hiroshige S, Ono E, Nishitake T, Ueno T, Miyazaki E, Kumamoto T. Reduced IgG levels found during acute eosinophilic pneumonia, which normalize during recovery from disease. Respir Med. 2008; 102:899–903.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download