INTRODUCTION
MATERIALS AND METHODS
Patient population
Data collection
Cytokine assay
Statistical analysis
RESULTS
Clinical features
Table 1
Demographic and clinical profiles of the study participants

Fig. 1

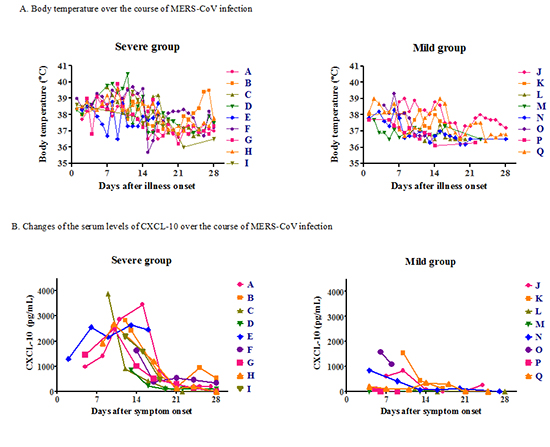
Cytokine profiles
Fig. 2

Fig. 3

Journal List > J Korean Med Sci > v.31(11) > 1023131




Funding This work was supported by a grant from the Korean Healthcare Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C3227).
AUTHOR CONTRIBUTION Conception and design: Oh MD, Kim ES, Choe PG, Park WB. Acquisition of data: Kim ES, Choe PG, Park WB, Oh HS, Kim EJ, Nam EY, Na SH, Kim MS, Song KH, Bang JH, Park SW. Analysis and interpretation of data: Oh MD, Kim ES, Choe PG, Park WB, Song KH, Bang JH, Park SW, Kim HB, Kim NJ. Manuscript preparation: Oh MD, Kim ES, Choe PG. Manuscript approval: all authors.
Eu Suk Kim 
https://orcid.org/http://orcid.org/0000-0001-7132-0157
Pyoeng Gyun Choe 
https://orcid.org/http://orcid.org/0000-0001-6794-7918
Wan Beom Park 
https://orcid.org/http://orcid.org/0000-0003-0022-9625
Hong Sang Oh 
https://orcid.org/http://orcid.org/0000-0002-4535-6305
Eun Jung Kim 
https://orcid.org/http://orcid.org/0000-0003-3302-7827
Eun Young Nam 
https://orcid.org/http://orcid.org/0000-0003-2488-3226
Sun Hee Na 
https://orcid.org/http://orcid.org/0000-0002-9605-7807
Moonsuk Kim 
https://orcid.org/http://orcid.org/0000-0001-8640-939X
Kyoung-Ho Song 
https://orcid.org/http://orcid.org/0000-0002-4517-3840
Ji Hwan Bang 
https://orcid.org/http://orcid.org/0000-0002-7628-1182
Sang Won Park 
https://orcid.org/http://orcid.org/0000-0002-0550-1897
Hong Bin Kim 
https://orcid.org/http://orcid.org/0000-0001-6262-372X
Nam Joong Kim 
https://orcid.org/http://orcid.org/0000-0001-6793-9467
Myoung-don Oh 
https://orcid.org/http://orcid.org/0000-0002-2344-7695