1. Yang BM, Kim DJ, Byun KS, Kim HS, Park JW, Shin S. The societal burden of HBV-related disease: South Korea. Dig Dis Sci. 2010; 55:784–793.
2. Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009; 15:S13–S24.
3. Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014; 384:2053–2063.
4. Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009; 15:S50–S59.
5. Lim YS, Suh DJ. Current antiviral therapy for chronic hepatitis B. J Korean Med Sci. 2004; 19:489–494.
6. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50:661–662.
7. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012; 57:167–185.
8. Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012; 6:531–561.
9. KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol. 2012; 18:109–162.
10. Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011; 141:1240–1248. 1248.e1–1248.e2.
11. Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010; 51:1577–1583.
12. Park HN, Sinn DH, Gwak GY, Kim JE, Rhee SY, Eo SJ, Kim YJ, Choi MS, Lee JH, Koh KC, et al. Upper normal threshold of serum alanine aminotransferase in identifying individuals at risk for chronic liver disease. Liver Int. 2012; 32:937–944.
13. Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007; 47:760–767.
14. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
15. Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008; 28:1067–1077.
16. Korean Liver Cancer Study Group. National Cancer Center (KR). Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009; 15:391–423.
17. Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014; 63:1943–1950.
18. Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010; 53:348–356.
19. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013; 381:468–475.
20. Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, Kim BS, Park YM, Suzuki S, Sugauchi F, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005; 20:816–820.
21. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, E DV, Vianello L, Zanuso F, Mozzi F, Milani S, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002; 137:1–10.
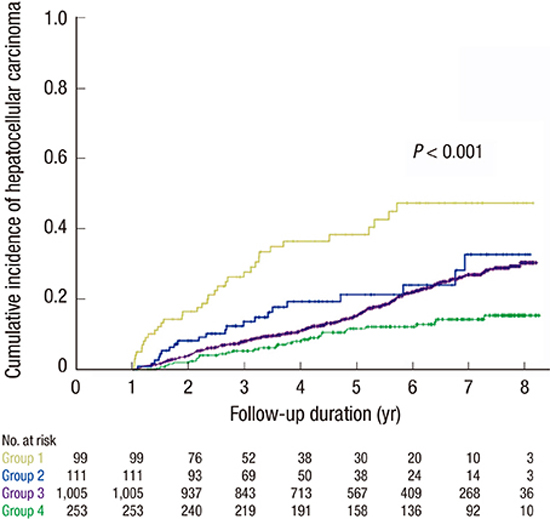








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download