Abstract
Therapeutic plasma exchange (TPE) is one possible treatment for patients resistant to conventional antithyroid drugs or requiring urgent attention for thyrotoxicosis. We report a 35-yr-old man with thyrotoxicosis, ultimately attributed to Graves' disease in whom antithyroid drug used initially was soon discontinued, due to abnormal liver function, and replaced by Lugol's solution. Three weeks later, an escape phenomenon (to Lugol's solution) was apparent, so we performed TPE to control the thyrotoxicosis. Two courses of TPE by a centrifugal type machine resulted in diminished levels of thyroid hormone levels, which then rebounded after another two courses of membrane filtration type TPE. However, the patient could be treated with radioactive iodine therapy without any complications at present.
In conventional management of Grave disease (GD), there are three major options: antithyroid drugs, radioactive iodine (RAI) therapy, and thyroidectomy (1). According to the guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists, any one of these modalities is acceptable, as dictated by physician's experience and/or patient preference; although a recent systematic review, based on specific outcomes, suggests that surgery may be the most effective (12).
In Asia and Europe, antithyroid drugs are preferred as first-line treatment of GD. However, serious related adverse effects (ie, agranulocytosis or hepatotoxicity) may ensue, in which case these drugs should be withdrawn, and RAI therapy or surgery should be considered (12). Since thyroid function should be at or near normal to avoid potential transient aggravation of thy-rotoxicosis during or after RAI or surgery (1), adjunctive management of thyrotoxicosis is often required. Iodine solution is the most widely used adjunct in this context, and it is usually quite effective in stabilizing thyroid function (1). Still, its effects are often transient and an escape phenomenon may develop with long-term use. In patients refractive to iodine solution, especially those requiring urgent surgery or those at risk of organ failure from thyrotoxicosis, an alternate modality is needed for appropriate control of thyroid function. Removal of circulating thyroid hormone by therapeutic plasma exchange (TPE) has been reported as a useful adjunct in these instances (345). We described a patient with GD who underwent successful sequential TPE and RAI therapy. The efficacy of TPE as an adjunct treatment in the management of GD is also reviewed.
A 35-yr-old man with chronic hepatitis-B viral infection presented to Seoul National University Hospital with a 15-day history of pruritus on April 2013. He also complained of tremor, dyspnea on exertion, and weight loss of 6 kg over the previous month. Baseline laboratory investigations resulted in a biochemical picture of hyperthyroidism: total T3, 270 ng/dL (87-184 ng/dL); free T4, 5.79 ng/dL (normal range, 0.70-1.80 ng/dL); TSH, < 0.05 µIU/mL (0.04-4.1 µIU/mL); and TSH receptor antibody, 39.70 IU/L (0-1 IU/L). A thyroid scan showed diffuse glandular enlargement, with a generalized increase in uptake of Tc-99m, further substantiating a clinical diagnosis of GD. Liver function tests (LFTs) were abnormal from one month previously) showing slight increases in transaminase levels (AST, 70 IU/L [0-40 IU/L]; ALT, 174 IU/L [0-40 IU/L]) and hyperbilirubinemia (total bilirubin, 1.8 mg/dL [0.2-1.2 mg/dL]). Based on the clinical diagnosis, methimazole (MMI) 40 mg/day was prescribed. In addition, 5 mg/day levocetirizine and 20 mg/day propranolol were given to alleviate pruritus and associated symptoms.
The next day, the patient presented to the emergency room, complaining of acute-onset abdominal pain and diarrhea. Even higher elevations of LFTs (AST, 94 IU/L; ALT, 191 IU/L) and total bilirubin (2.5 mg/dL) were found on laboratory testing, with normal white blood cell (5.16×103/µL [4-10×103/µL]) and differential counts. The cause of such LFT abnormalities was difficult to ascertain, given that thyrotoxicosis alone, MMI-induced toxicity, and other factors were all potential etiologies. As a precaution, MMI was withdrawn immediately, and 2 drops Lugol's solution t.i.d was prescribed. Nevertheless, abnormal LFTs persisted (AST, 99 IU/L; ALT, 168 IU/L; total bilirubin, 6.0 mg/dL) by day 11 of hospitalization (Table 1). At the same time, the possibility of a diagnosis of autoimmune hepatitis (AIH) or a flare of viral hepatitis was entertained. Testing for antimitochondrial and anti-smooth muscle antibodies was negative, whereas assay of fluorescent antinuclear antibody proved positive (FANA: 1:40, speckled pattern). A liver biopsy disclosed histologic features compatible with mixed viral and cholestatic hepatitis, but not AIH. Resistance to the anti-HBV agents, lami-vudine and adefovir, was also problematic in this patient, calling for a change in medication to 300 mg tenofovir and 1 mg entecavir. The total bilirubin level increased to 9.5 U/L after 2 weeks of the new antiviral regimen, so 30 mg prednisolone was added with potential AIH in mind. Interestingly, the LFTs improved immediately once prednisolone was started (Table 1).
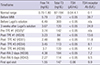
In the 3 weeks during which Lugol solution was given, serum levels of free T4 and T3 declined gradually to 3.67 ng/dL and 120 ng/dL by day 24 of hospitalization (Table 2). However, by day 25, both parameters rebounded (free T4, 3.74 ng/dL; T3, 142 ng/dL), suggesting an evolving refractory or escape phenomenon related to Lugol solution (Table 2). Due to the abnormal LFTs, antithyroid drugs were not an option, so RAI treatment was the next resort. Unfortunately, the free T4 level was still elevated (3.74 ng/dL) and 2 weeks of iodine restriction was required prior to RAI treatment, so another strategy was needed to correct the thyroid function. Consequently, 4 courses of TPE were performed via right jugular vein catheterization. The first two courses involved a centrifuge-driven cell separator (Cobe Spectra; Terumo BCT, Lakewood, CO, USA), which was not available thereafter because other patient was occupying the machine. A membrane filtration unit (Plasauto EZ Asahi Plasmaflo OP-05W; Asahi Kasei Medical, Tokyo, Japan) was instead used for the final two courses. ACD-A (with centrifugation) and heparin (with filtration) were both used as anticoagulants, depending on the occasion. Approximately 3 L of plasma was extracted on each procedure and replaced with 4% albumin, monitoring vital signs and adverse events in the process. Serum levels of free T4 and T3 were determined after each TPE session. Following the first two courses of TPE by centrifugation, free T4 level decreased by 33.7%; however, free T4 level increased by 49.2% after the final two courses of TPE by filtration (Table 2). RAI treatment at a dose of 15 mCi, was administered as planned after the fourth course of TPE, without any adverse consequences. Serum levels of free T4 (4.38 ng/dL) and T3 (184 ng/dL) were transiently elevated after RAI treatment but started to fall by day 42 of hospitalization (free T4, 2.27 ng/dL; T3, 114 ng/dL) (Table 2). The LFT values were also much improved (AST, 47 IU/L; ALT, 55 IU/L; total bilirubin, 4.8 mg/dL), and the patient was discharged on an anti-HBV regimen plus prednisolone (tapering by 5 mg/week) (Table 1). Levels of thyroid hormone levels were normalized one month after RAI treatment (Table 2).
TPE is a highly effective blood purification procedure used to treat a variety of disorders, such as autoimmune diseases or severe sepsis (67). It is a safe and useful process whereby blood is separated via centrifugation or filtration into plasma and cells. The cells are then returned to the patient, replacing the plasma with either donor plasma, fresh frozen plasma (FFP), albumin, or a similar colloidal solution (6). Because the therapeutic goal of TPE is the removal of harmful substances such as immune complexes, cytokines, toxins, or hormones, patients with severe hyperthyroidism stand to benefit from this procedure (46). The use of TPE for controlling thyrotoxicosis was first reported in the 1970s (8). Three thyrotoxic patients, refractory to conventional antithyroid agents, responded well to TPE and ultimately underwent RAI therapy. Likewise, refractory thyrotoxicosis was a primary indication for TPE in other earlier reports (349). Two to five courses of TPE were administered in patients with GD or toxic nodular goiter where thyroidal hyperfunction was not controlled by antithyroid drugs. In some instances, TPE was employed when antithyroid drugs were contraindicated, due to such adverse effects as agranulocytosis or hepatotoxicity (591011).
One retrospective multicenter study analyzed outcomes of TPE in a relatively large number of thyrotoxic patients (4). When TPE was administered to 22 patients with thyrotoxicosis, 9 with GD, and 13 with toxic nodules, clinical improvement was observed in most (20/22; 91%). A mean of four TPE courses (range, 2-9 courses) were administered before proceeding with RAI therapy or surgery, with free T4 levels declining by 41.7%. Our patient displayed a modest reduction in free T4 level after the initial two courses of TPE (33.7% reduction in free T4 from pre-TPE baseline). However, a rebound in thyroid hormone levels occurred after the next two courses. Of note, the type of TPE unit had changed from centrifugal (initial two courses) to membrane filtration (subsequent courses) during the treatment period. Previous reports indicate declines of 10%-20% in free T4 levels after each round of TPE, regardless of whether centrifugal (4911) or membrane filtration (35) unit were used. Centrifugation requires less blood volume and blood flow rate to achieve similar plasma removal (6) and thus may be more efficient than membrane filtration. However, no comparison studies have been done to directly evaluate these two methods. To the best of our knowledge, this is the first instance where both methods of TPE have been applied in the same patient. Although our findings clearly require corroboration through further study, centrifugation seemed superior to membrane filtration in controlling thyrotoxicosis. On the other hand, it may well be that thyrotoxicosis in this patient was increasing, making it more difficult for levels of thyroid hormone to be corrected during the latter two courses of TPE, even with equivalent performance by both machines.
Patients generally should be rendered euthyroid before RAI therapy or surgery to prevent the adverse effects of hyperthyroidism (121314). Although, conventional preparations, including antithyroid drugs, iodine, lithium, and corticosteroids are often useful (11516), such treatments are sometimes disappointing, as in this case. TPE may be considered as a bridge to other remedies when thyrotoxicosis cannot be controlled medically or if urgent symptomatic relief from thyrotoxicosis is needed (9). Our patient was accepted for TPE because there was no evident contraindication to TPE such as hemodynamic instability, active infection, bleeding tendency or allergic reaction to FFP or albumin (17). Finally, RAI treatment proceeded as planned, even though the levels of thyroid hormone levels did not normalize. He had no symptoms related to thyrotoxicosis and his thyroid hormone levels were not excessive (i.e., more than three times the upper normal limit), so RAI therapy was not condtraindicated (1). Thus, TPE helped in transitioning to RAI treatment, without worsening of thyrotoxicosis and/or provoking related complications.
In summary, we were able to adequately stabilize our patient's thyroid function using TPE, enabling us to proceed with RAI therapy as a more definitive treatment of GD. Because TPE provides only transient improvement in thyroid function, several courses are mandatory. Our data suggest that TPE is an effective temporizing measure for patients with GD in the event that all other conventional treatment modalities prove unsuitable or ineffective.
Figures and Tables
Table 1
Liver function tests on admission, after change in medication, and after each course of plasmapheresis
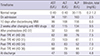
Table 2
Thyroid hormone levels before and after therapeutic plasmapheresis
References
1. Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, et al. American Thyroid Association. American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011; 17:456–520.
2. Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for Graves' disease? A systematic review of the existing literature. Ann Surg Oncol. 2013; 20:660–667.
3. Pasimeni G, Caroli F, Spriano G, Antonini M, Baldelli R, Appetecchia M. Refractory thyrotoxicosis induced by iodinated contrast agents treated with therapeutic plasma exchange. A case report. J Clin Apher. 2008; 23:92–95.
4. Keklik M, Kaynar L, Yilmaz M, Sivgin S, Solmaz M, Pala C, Aribas S, Akyol G, Unluhizarci K, Cetin M, et al. The results of therapeutic plasma exchange in patients with severe hyperthyroidism: a retrospective multicenter study. Transfus Apher Sci. 2013; 48:327–330.
5. Lew WH, Chang CJ, Lin JD, Cheng CY, Chen YK, Lee TI. Successful preoperative treatment of a Graves' disease patient with agranulocytosis and hemophagocytosis using double filtration plasmapheresis. J Clin Apher. 2011; 26:159–161.
6. Ward DM. Conventional apheresis therapies: a review. J Clin Apher. 2011; 26:230–238.
7. Venkataraman R, Subramanian S, Kellum JA. Clinical review: extracorporeal blood purification in severe sepsis. Crit Care. 2003; 7:139–145.
8. Ashkar FS, Katims RB, Smoak WM 3rd, Gilson AJ. Thyroid storm treatment with blood exchange and plasmapheresis. JAMA. 1970; 214:1275–1279.
9. Ezer A, Caliskan K, Parlakgumus A, Belli S, Kozanoglu I, Yildirim S. Preoperative therapeutic plasma exchange in patients with thyrotoxicosis. J Clin Apher. 2009; 24:111–114.
10. Miljić D, Stojanović M, Ješić R, Bogadnović G, Popović V. Role of plasma exchange in autoimmune hyperthyroidism complicated by severe tiamazol-induced cholestatic jaundice. Transfus Apher Sci. 2013; 49:354–356.
11. Aydemir S, Ustundag Y, Bayraktaroglu T, Tekin IO, Peksoy I, Unal AU. Fulminant hepatic failure associated with propylthiouracil: a case report with treatment emphasis on the use of plasmapheresis. J Clin Apher. 2005; 20:235–238.
12. Cooper DS. Hyperthyroidism. Lancet. 2003; 362:459–468.
13. Fisher JN. Management of thyrotoxicosis. South Med J. 2002; 95:493–505.
14. Houghton SG, Farley DR, Brennan MD, van Heerden JA, Thompson GB, Grant CS. Surgical management of amiodarone-associated thyrotoxicosis: Mayo Clinic experience. World J Surg. 2004; 28:1083–1087.
15. Lazarus JH, Richards AR, Addison GM, Owen GM. Treatment of thyrotoxicosis with lithium carbonate. Lancet. 1974; 2:1160–1163.
16. Bogazzi F, Bartalena L, Brogioni S, Scarcello G, Burelli A, Campomori A, Manetti L, Rossi G, Pinchera A, Martino E. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves' hyperthyroidism. J Clin Endocrinol Metab. 1999; 84:499–503.
17. Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: complications and management. Am J Kidney Dis. 1994; 23:817–827.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download