Abstract
Although extended-spectrum β-lactamase-producing Escherichia coli (ESBL-EC) has emerged as a significant community-acquired pathogen, there is little epidemiological information regarding community-onset bacteremia due to ESBL-EC. A retrospective observational study from 2006 through 2011 was performed to evaluate the epidemiology of community-onset bacteremia caused by ESBL-EC. In a six-year period, the proportion of ESBL-EC responsible for causing community-onset bacteremia had increased significantly, from 3.6% in 2006 to 14.3%, in 2011. Of the 97 clinically evaluable cases with ESBL-EC bacteremia, 32 (33.0%) were further classified as healthcare-associated infections. The most common site of infection was urinary tract infection (n=35, 36.1%), followed by biliary tract infections (n=29, 29.9%). Of the 103 ESBL-EC isolates, 43 (41.7%) produced CTX-M-14 and 36 (35.0%) produced CTX-M-15. In the multilocus sequence typing (MLST) analysis of 76 isolates with CTX-M-14 or -15 type ESBLs, the most prevalent sequence type (ST) was ST131 (n=15, 19.7%), followed by ST405 (n=12, 15.8%) and ST648 (n=8, 10.5%). No significant differences in clinical features were found in the ST131 group versus the other group. These findings suggest that epidemic ESBL-EC clones such as CTX-M-14 or -15 type ESBLs and ST131 have disseminated in community-onset infections, even in bloodstream infections, which are the most serious type of infection.
Escherichia coli is a common cause of urinary tract infection (UTI) and intra-abdominal infection in humans of all ages, and the spectrum of pathology can range from a cystitis to life-threatening sepsis syndrome (1). The prevalence of resistance to fluoroquinolones and extended-spectrum cephalosporins in E. coli has increased dramatically over the past decade (2). We have witnessed that plasmid-mediated extended-spectrum β-lactamases (ESBLs) have become prominent in community-onset E. coli infections, as well as nosocomial infections. This increase is largely the result of the widespread emergence of a single disseminated E. coli clonal group, designated sequence type (ST) 131 on the basis of multilocus sequence typing (MLST) (1-3). Since ESBL-producing organisms are frequently resistant to multiple antimicrobial agents, therapeutic options for these infections are severely limited.
Numerous studies have been published that describe the epidemiology and molecular characterization of the ESBLs (2-12); however, few studies have presented sufficient clinical data establishing a relationship between the ESBL type or ST and clinical characteristics, particularly in community-onset E. coli bacteremia, the most serious type of infection. Moreover, clinically oriented data regarding community-onset infections caused by ESBL-producing E. coli (ESBL-EC) are limited, and most studies included patients with UTIs involving ESBL-producing isolates. There is little information about the risk factors and treatment outcomes in cases of community-onset bacteremia due to ESBL-EC. In this study, we evaluated community-onset bacteremia cases due to ESBL-EC that occurred over a six-year period and were not associated with an outbreak of such infections. The primary objectives of this study were to investigate the microbiologic characteristics and molecular epidemiology of community-onset bacteremia caused by ESBL-EC and to delineate their clinical features.
A retrospective observational study was performed to evaluate the epidemiology of community-onset bacteremia caused by ESBL-EC. We reviewed the medical records of individuals diagnosed with E. coli bacteremia from January 2006 through December 2011 at Samsung Medical Center (a 1,950-bed tertiary care university hospital in Seoul, Korea). Patients were included in the study if their blood cultures were drawn in the Emergency Department within 48 hr of admission and were positive for E. coli. All patients aged >15 yr were enrolled and only the first bacteremic episode for each patient was included in the analysis.
The data collected included age, gender, underlying diseases, site of infection, and antimicrobial regimen. The presence of the following comorbid conditions was also documented: recent surgical procedure, corticosteroid use, immunosuppressant use, presence of an indwelling urinary catheter or a percutaneous tube. Since this study was observational, patient management and the antimicrobial treatment regimen was decided upon by the patients' physicians without any guidelines or intervention from the study investigators.
E. coli bacteremia was defined as the presence of E. coli in the blood, documented by at least one positive blood culture. The site of infection was determined by the patients' physicians on the basis of E. coli isolation from the presumed portal of entry and a clinical evaluation. Community-onset infection was defined as an infection diagnosed within the first 48 hr of hospitalization. Since many cases of bacteremia that are present or incubating upon admission to the hospital are nonetheless healthcare-associated, we refer to non-nosocomial bacteremia as community-onset rather than community-acquired (13). Episodes of community-onset bacteremia were further classified as healthcare-associated if any of the following criteria were present: history of hospital admission of >48 hr duration in the previous 90 days, hemodialysis, intravenous medication, home wound care in the previous 30 days, or residence in a nursing home or long-term care facility (13). When cases did not meet these criteria, they were categorized as community-associated. Severe sepsis was defined as sepsis with one or more clinical signs of organ dysfunction.
Of the stored blood isolates collected by the clinical microbiology laboratory in our hospitals, 103 ESBL-EC isolates were successfully recovered for inclusion in the study. Species identification was performed using the standard VITEK II identification card (bioMerieux, Hazelwood, MO, USA). Antimicrobial susceptibility testing and ESBL confirmatory testing were performed using the broth microdilution method, following the recommendations of the Clinical and Laboratory Standards Institute (CLSI). The minimum inhibitory concentrations (MICs) of the antimicrobial compounds tested were determined by the broth microdilution method. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality-control strains.
The ESBL-related genes, including TEM, SHV, CTX-M, and OXA, were amplified by polymerase chain reaction (PCR) from clinical isolates as described previously (6, 14, 15). PCR and sequencing of PCR products were performed to identify the blaTEM, blaSHV, and blaCTX-M genes responsible for the ESBL activity in the ESBL-producing strains. Both strands of all PCR fragments were sequenced and the types of β-lactamase genes were identified by comparing the sequences against those in the database of G. Jacoby and K. Bush (http://www.lahey.org/Studies/). MLST was performed by following the method of M. Achtamn and colleagues (http://mlst.ucc.ie/mlst/dbs/Ecoli), in which seven housekeeping genes, adk (adenylate kinase), fumC (fumarate hydratase), gyrB (DNA gyrase), icd (isocitrate dehydrogenase), mdh (malate dehydrogenase), purA (adenylosuccinate synthetase), and recA (ATP/GTP binding motif), were used (16, 17). Clonal complexes were followed by those of the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli).
Student's t-tests were used to compare continuous variables, and the chi-squared or Fisher's exact tests were used to compare categorical variables. All P values were two-tailed, and P values of <0.05 were considered to be statistically significant. The SPSS statistical software for Windows, PASW version 18.0, was used for the statistical analyses.
During a six-year period (2006 to 2011), a total of 1,517 community-onset E. coli bacteremia episodes were identified, of which 160 (10.6%) were ESBL-EC bacteremic episodes. The proportion of ESBL-EC-caused community-onset bacteremia had increased significantly, from 3.6% in 2006 to 14.3% in 2011 (Fig. 1). Of the 160 ESBL-producing isolates, 103 non-duplicated isolates were collected for further microbiologic analysis, and a total of 97 patients with community-onset ESBL-EC bacteremia were included in the clinical analysis. Of these 97 cases, 32 (33.0%) were further classified as healthcare-associated infections, and the remaining 65 (67.0%) as community-associated infections. The mean patient age was 59.2 yr (standard deviation [SD], 16.7 yr), and 55 (56.7%) of the patients were males. The most common underlying diseases were solid tumors (n=49, 50.5%), followed by diabetes mellitus (DM, n=29, 29.9%) and liver diseases (n=19, 19.6%). The most common sites of infection were the urinary tract (n=35, 36.1%), followed by the biliary tract (n=29, 29.9%). The clinical characteristics and predisposing factors of community-onset bacteremia caused by ESBL-EC are shown in Table 1. The majority of patients (n=59, 60.8%) received prior antimicrobial therapy, primarily cephalosporins (n=47, 48.5%). The overall 30-day mortality was 12.6% (12/95).
Of the 103 ESBL-EC isolates that caused community-onset bacteremia, 43 (41.7%) produced CTX-M-14, 36 (35.0%) produced CTX-M-15, and 24 (23.3%) produced other types of ESBL (CTX-M-24, 9 isolates; CTX-M-27, 8 isolates; CTX-M-3, 4 isolates; CTX-M-57, 3 isolates). Fifty-seven isolates (55.3%) also produced TEM-1 in addition to CTX-M-type ESBL, and 2 isolates produced TEM-15 or SHV-11 in addition to CTX-M-14. The distribution of CTX-M-type ESBL genes over the six-year period is shown in Fig. 2. The most common ESBL types were CTX-M-14 and CTX-M-15 for each year.
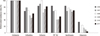
Resistance rates to various antimicrobial agents are shown in Table 2. Of the 103 isolates included in this study, 72 (69.9%) were non-susceptible to ciprofloxacin, 50 (48.5%) were non-susceptible to piperacillin-tazobactam, and 81 (78.6%) were non-susceptible to cefepime. While no resistance to meropenem or imipenem was detected using the 2011 CLSI breakpoints, 11 (10.7%) were non-susceptible to ertapenem. The MIC50 and MIC90 values of antimicrobial agents were higher in CTX-M-15 producers than in CTX-M-14 producers (Table 2). The change in the antimicrobial resistance rates of the ESBL-EC isolates over the six-year period was shown in Fig. 3.
In the MLST analysis, 23 different STs were identified among 76 isolates with CTX-M-14 or -15 type ESBLs. The most prevalent ST was ST131 (n=15, 19.7%), followed by ST405 (n=12, 15.8%) and ST648 (n=8, 10.5%). The distribution of STs among the CTX-M-14 or -15-type ESBL-EC isolates was shown in Table 3.
The clinical characteristics of community-onset bacteremia due to CTX-M-15 type ESBL-EC isolates were compared with those of others. Old age was more common in the CTX-M-15 group than in the other group (57.6% vs 33.9%; P=0.028). Biliary tract infection was the most common site of infection in the CTX-M-15 group, while UTI was the most common site of infection in the other group (Table 4). No significant differences in underlying diseases or comorbid conditions were found between the two groups (Table 4). The clinical characteristics of community-onset bacteremia due to ST131 ESBL-EC isolates were also compared with those of others. UTI was the most common site of infection among ST131 isolates. No significant differences in clinical features were found among the two groups (Table 5).
This study investigated the clinical and microbiological characteristics of ESBL-EC isolates responsible for causing community-onset bacteremia over a six-year period (2006-2011) in Korea, and showed that these isolates have increased significantly during the study period. Several worldwide reports have reported the emergence of CTX-M-producing E. coli as a significant pathogen since the turn of the 21st century (1, 18, 19). MLST is an excellent tool for evolutionary studies, which can show common ancestry lineages among bacteria, and it has led to the definition of major sequence types and the recognition of clones that have successfully spread internationally, such as ST131 (18-20). We have identified several major MLST STs, ST38, ST69, ST95, ST131, ST405, and ST648. ST131 is a major drug-resistant pathogen among the ESBL-EC strains, similarly found in different parts of the world, including Korea, and poses an important new public health threat within our region (5, 17, 18). As expected, CTX-M-14 and -15 were the predominant types of ESBL identified, and ST131 was the most common among those isolates. To our knowledge, this is the first large study of community-onset ESBL-EC bacteremia to describe detailed clinical and microbiological characteristics. Similar to the global spread, ST131 has emerged as the most predominant isolate in our cohort of community-onset ESBL-EC bacteremia cases.
Several worldwide reports have shown that CTX-M-producing E. coli isolates are important causes of bloodstream infections, with the urinary tract as the most frequent infection site (4, 9, 18). Our study provides a comprehensive analysis of community-onset bacteremia caused by ESBL-EC, and the distribution by type of infection was as expected for E. coli, in that UTIs accounted for the majority of cases (36.1%). However, it is noteworthy that 41.2% of the cases had biliary tract infections or intra-abdominal infections. The frequency of intra-abdominal infections, in which ESBL-EC are involved, is probably underestimated, since samples from patients with community-acquired secondary peritonitis are not routinely sent for culturing (8, 21). Our data suggest that the notion that community-acquired ESBL-EC infections are more likely to involve infections of the urinary tract may not be applicable to E. coli bacteremia, which is one of the most serious types of infection. Notably, our previous studies demonstrated that the most common sites of infection in community-onset ESBL-EC bacteremia are intra-abdominal infections, including biliary tract infection as well as UTIs (22, 23). CTX-M-15-type ESBL-EC isolates and ST131 isolates had a broad spectrum of infection syndromes, including UTI, biliary tract infection, and intra-abdominal infections.
We have previously reported that two major clones of ciprofloxacin-resistant E. coli isolates responsible for causing UTIs in our community were ST131 and ST393 (17). This current study focusing on community-onset bacteremia caused by ESBL-EC, not just UTIs, suggests that ST131- or CTX-M-15-type ESBL-EC isolates may have disseminated in the Korean community and have become a significant threat to public health. CTX-M-15 type ESBL-EC isolates had a significant association with resistance to key first-line antimicrobial therapies, notably β-lactams and fluoroquinolones. A previous study showed that, when compared with patients with non-ST131 infections, those with clone ST131 were more likely to have secondary bacteremia and non-catheterized UTIs (4). Although more virulence factors and antimicrobial resistance were observed with the ST131 isolate, patients with the ST131 isolate did not experience worse outcomes than patients with non-ST 131 isolates (4). No significant differences in clinical features were found among the two groups in our study.
Our study has several limitations. First, clinical data were retrospectively collected through electronic medical records and chart review, although cases and bacterial isolates were enrolled prospectively. The present study was observational, and thus, the possibility of limitations that preclude accurate comparisons should be kept in mind, given the observational nature of this study. Second, the clinical data on ST131 were limited to patients with CTX-14- or -15-type ESBL-EC, and we do not know whether ST131 would exhibit similar risk factors and clinical features in other type of ESBL-EC or non-ESBL-EC infections. Study limitations included a lack of information regarding the identity of the non-CTX-M-14 or -15 ESBLs. Finally, data were obtained from a single center in Korea. Strengths included the large sample size relative to other studies of community-onset ESBL-EC bacteremia, long study interval, analysis of clinical characteristics, and extensive molecular characterization of the isolates.
In conclusion, this study confirms that ESBL-EC isolates are a notable cause of community-onset bacteremia in predisposed patients. The widespread and rapid dissemination of ESBL-EC seems to be an emerging issue worldwide, and ESBL-EC is a pathogen that is increasingly found in the community, and that may drive significant changes in the empirical use of antibiotics for certain infections. Our data suggest that epidemic ESBL-EC clones, such as CTX-M-14- or -15-type ESBLs and ST131, have become disseminated in community-onset infections including in bloodstream infections. Further clinical and epidemiologic studies are needed to guide clinicians in the management of community-onset infections caused by E. coli.
Figures and Tables
Fig. 1
Annual incidence and proportion of ESBL-producing E. coli isolates causing community-onset bacteremia (Gray bar, number; black line, prevalence).
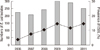
Fig. 2
Change in the ESBL prevalence (%) and the types of CTX-M genes during six years of E. coli-related community-onset bacteremia cases. *Others: CTX-M-3, CTX-M-24, CTX-M-27, and CTX-M-57.

Table 1
Clinical characteristics and predisposing factors of community-onset bacteremia caused by ESBL-producing E. coli bacteremia
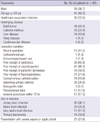
Table 3
Distribution of STs among CTX-M-14- and -15-producing E. coli isolates over the period of 2006 to 2011

ACKNOWLEDGMENTS
We would like to thank Mi Young Lee for technical support and Kwi Suk Yoon for clinical data collection. Bacterial isolates were obtained from the Asian Bacterial Bank (ABB) of the Asia Pacific Foundation for Infectious Diseases (APFID).
References
1. Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother. 2011; 66:1–14.
2. Johnson JR, Nicolas-Chanoine MH, DebRoy C, Castanheira M, Robicsek A, Hansen G, Weissman S, Urban C, Platell J, Trott D, et al. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967-2009. Emerg Infect Dis. 2012; 18:598–607.
3. Croxall G, Hale J, Weston V, Manning G, Cheetham P, Achtman M, McNally A. Molecular epidemiology of extraintestinal pathogenic Escherichia coli isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial resistance in both community and hospital care settings. J Antimicrob Chemother. 2011; 66:2501–2508.
4. Chung HC, Lai CH, Lin JN, Huang CK, Liang SH, Chen WF, Shih YC, Lin HH, Wang JL. Bacteremia caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob Agents Chemother. 2012; 56:618–622.
5. Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS 2nd, Hansen G, Edelstein PH, Robicsek A, Cleary T, Adachi J, et al. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-β-lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob Agents Chemother. 2012; 56:2364–2370.
6. Khanna N, Boyes J, Lansdell PM, Hamouda A, Amyes SG. Molecular epidemiology and antimicrobial resistance pattern of extended-spectrum-β-lactamase-producing Enterobacteriaceae in Glasgow, Scotland. J Antimicrob Chemother. 2012; 67:573–577.
7. Peirano G, Sang JH, Pitondo-Silva A, Laupland KB, Pitout JD. Molecular epidemiology of extended-spectrum-β-lactamase-producing Klebsiella pneumoniae over a 10 year period in Calgary, Canada. J Antimicrob Chemother. 2012; 67:1114–1120.
8. Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008; 168:1897–1902.
9. Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Ruíz M, Peña C, Almela M, Almirante B, Grill F, Colomina J, et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010; 50:40–48.
10. Van der Bij AK, Peirano G, Goessens WH, van der Vorm ER, van Westreenen M, Pitout JD. Clinical and molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam Area, Netherlands. Antimicrob Agents Chemother. 2011; 55:3576–3578.
11. Peirano G, Costello M, Pitout JD. Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX-M-15 in community hospitals. Int J Antimicrob Agents. 2010; 36:19–23.
12. Park SH, Choi SM, Lee DG, Kim J, Choi JH, Kim SH, Kwon JC, Yoo JH. Emergence of extended-spectrum β-lactamase-producing escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. 2011; 17:537–544.
13. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, et al. Health care: associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797.
14. Kim J, Lim YM, Jeong YS, Seol SY. Occurrence of CTX-M-3, CTX-M-15, CTX-M-14, and CTX-M-9 extended-spectrum beta-lactamases in Enterobacteriaceae clinical isolates in Korea. Antimicrob Agents Chemother. 2005; 49:1572–1575.
15. Kim J, Lim YM, Rheem I, Lee Y, Lee JC, Seol SY, Lee YC, Cho DT. CTX-M and SHV-12 beta-lactamases are the most common extended-spectrum enzymes in clinical isolates of Escherichia coli and Klebsiella pneumoniae collected from 3 university hospitals within Korea. FEMS Microbiol Lett. 2005; 245:93–98.
16. Lau SH, Reddy S, Cheesbrough J, Bolton FJ, Willshaw G, Cheasty T, Fox AJ, Upton M. Major uropathogenic Escherichia coli strain isolated in the northwest of England identified by multilocus sequence typing. J Clin Microbiol. 2008; 46:1076–1080.
17. Lee MY, Choi HJ, Choi JY, Song M, Song Y, Kim SW, Chang HH, Jung SI, Kim YS, Ki HK, et al. Dissemination of ST131 and ST393 community-onset, ciprofloxacin-resistant Escherichia coli clones causing urinary tract infections in Korea. J Infect. 2010; 60:146–153.
18. Peirano G, van der Bij AK, Gregson DB, Pitout JD. Molecular epidemiology over an 11-year period (2000 to 2010) of extended-spectrum β-lactamase-producing Escherichia coli causing bacteremia in a centralized Canadian region. J Clin Microbiol. 2012; 50:294–299.
19. Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents. 2010; 35:316–321.
20. Sullivan CB, Diggle MA, Clarke SC. Multilocus sequence typing: data analysis in clinical microbiology and public health. Mol Biotechnol. 2005; 29:245–254.
21. Kang CI, Wi YM, Lee MY, Ko KS, Chung DR, Peck KR, Lee NY, Song JH. Epidemiology and risk factors of community onset infections caused by extended-spectrum β-lactamase-producing Escherichia coli strains. J Clin Microbiol. 2012; 50:312–317.
22. Kang CI, Cheong HS, Chung DR, Peck KR, Song JH, Oh MD, Choe KW. Clinical features and outcome of community-onset bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Eur J Clin Microbiol Infect Dis. 2008; 27:85–88.
23. Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Ki HK, Son JS, Lee SS, Kim YS, et al. Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2010; 36:284–287.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download