Abstract
Interstitial lung disease in children (chILD) is a group of disorders characterized by lung inflammation and interstitial fibrosis. In the past recent years, we noted an outbreak of child in Korea, which is possibly associated with inhalation toxicity. Here, we report a series of cases involving toxic inhalational injury-associated chILD with bronchiolitis obliterans pattern in Korean children. This study included 16 pediatric patients confirmed by lung biopsy and chest computed tomography, between February 2006 and May 2011 at Asan Medical Center Children's Hospital. The most common presenting symptoms were cough and dyspnea. The median age at presentation was 26 months (range: 12-47 months), with high mortality (44%). Histopathological analysis showed bronchiolar destruction and centrilobular distribution of alveolar destruction by inflammatory and fibroproliferative process with subpleural sparing. Chest computed tomography showed ground-glass opacities and consolidation in the early phase and diffuse centrilobular nodular opacity in the late phase. Air leak with severe respiratory difficulty was associated with poor prognosis. Although respiratory chemicals such as humidifier disinfectants were strongly considered as a cause of this disease, further studies are needed to understand the etiology and pathophysiology of the disease to improve the prognosis and allow early diagnosis and treatment.
Interstitial lung disease (ILD) (1) is a heterogeneous group of disorders that have similar clinical and radiological features and share the common histological characteristics of lung inflammation (2). From 2006 to 2011 in Korea, several infants and young children, who initially presented with cough in spring experienced rapidly progressive respiratory failure with pulmonary fibrosis (3, 4). These cases were characterized by bronchiolar destruction and rapid progression to pulmonary fibrosis with high mortality.
Most of our patients showed clinical and radiological features similar to those of acute interstitial pneumonia (AIP). However, in cases where the causative viral agent was not typical of AIP, histopathological and radiological findings were distinct as well. The disease could also be distinguished from hypersensitivity pneumonitis (HP) in its clinicopathologic features and response to treatment (5) (e-Table 1). Initially, we termed this type of interstitial pneumonia as "rapidly progressive bronchiolitis obliterans interstitial pneumonia in children" because it showed different clinical course, pathologic, and radiologic findings from those of other chILD subtypes. However, inhalation of humidifier disinfectants has been suggested as a risk factor for chILD with features similar to ours (6, 7).
To aid physicians in the diagnosis of this subtype of chILD, we herein provide a detailed description of the clinical, pathologic, and radiologic features of chILD induced by toxic inhalation at one medical center over a 5-yr period.
We reviewed the medical records of 51 pediatric patients who had been hospitalized with chILD associated with toxic inhalation between February 2006 and May 2011 at the Asan Medical Center Children's Hospital in Seoul, Korea. Among these 51 cases, 16 biopsy-proven cases of chILD associated with toxic inhalation were selected and enrolled in our study to clarify the diagnosis of the same clinical and pathologic features in these cases. The diagnosis was based on the clinical manifestations of ILD, namely acute onset of dyspnea, dry cough, or tachypnea with radiological findings of ground-glass opacities. The diagnosis was confirmed by histological analysis of the area of the lung with maximum infiltration, as indicated by chest computed tomography (CT) imaging. The lung tissue was obtained by video-assisted thoracoscopic surgery (VATS) in 15 cases and at autopsy in 1 case.
Patients' records, results of radiologic studies, and pathology specimens were reviewed to identify patients who presented with the following inclusion criteria: 1) rapidly progressive clinical course (< 2 months) of tachypnea, dyspnea, or respiratory difficulty; 2) bronchiolar destruction with alveolar edema fluid in the early phase, and spread of septal inflammatory and fibroblastic proliferation to the parenchymal area with relative subpleural sparing in the late phase on lung biopsy; 3) groundglass opacities, consolidation, or centrilobular nodular opacities on chest CT images; and 4) absence of chronic lung diseases such as preexisting ILD or other combined diseases.
Pathologic classification was reached on the basis of consensus between 2 expert pathologists who classified the lung specimens as early and late phase according to the criteria for the diagnosis of one of the subtypes of chILD. The chest radiography and CT images were reviewed by two radiologists. All patients underwent tests for the presence of respiratory syncytial virus, adenovirus, influenza virus, parainfluenza virus, cytomegalovirus, Epstein-Barr virus, and Mycoplasma pneumoniae. Laboratory tests for collagen vascular disease yielded negative results in all of the patients.
Fisher's exact test or the chi-square test was used to compare the clinical features, characteristics, outcome, and pathologic findings of the children who survived with those who died. All analyses were performed with SPSS version 18.0 for Window. A P value of less than 0.05 was considered significant.
The patients consisted of nine boys and seven girls. Of the 16, five patients were reported as familial cases. The age at presentation ranged from 12 to 47 months. The toxic inhalational lung injury associated interstitial lung disease occurred from early spring to early summer, with its peak prevalence in April (38%). The demographic characteristics are summarized in Table 1.
The most common symptom was cough followed by dyspnea and tachypnea in six patients (38%) as shown in Table 2. There was considerable variation in the severity of the signs and symptoms. Fever (≥ 38℃) was recorded in two patients (13%), while hypoxemia at room air was recorded in 15 patients (94%). The clinical characteristics are summarized in Table 3. The median time between symptom onset and diagnostic confirmation by biopsy for 15 of the cases was 23 days. The median time until hospitalization after symptom onset was 22 days. CT scanning was performed a mean of 4 days prior to biopsy. All of the seven patients who required mechanical ventilation for acute respiratory failure were died (P = 0.001). The mean duration of mechanical ventilation was 54 days. Pulmonary function tests could not be performed.
The patients diagnosed with this disease commonly present with prodromal symptoms such as cough for 2-3 weeks, followed by rapid progression to respiratory failure with hypoxemia on room air despite active treatment. This disease has a propensity to develop during spring and shows rapid progression in its course with high mortality.
The pathologic diagnosis was made by lung biopsy through VATS in 15 patients and by autopsy in 1 patient. No evidence of viral, bacterial, or fungal infection was found in the pathology specimens. The pathologic characteristics were bronchiolar destruction accompanied by mild to severe bronchiolar obliteration mimicking constrictive and obliterative bronchiolitis, with a predominantly centrilobular distribution of alveolar destruction by inflammatory cell infiltration and fibroblastic proliferation (Fig. 1). In most cases, the fibroinflammatory process was temporally homogeneous and spatially heterogeneous. The above features contrasted with those of the typical diffuse alveolar damage (DAD). A multifocal foamy histiocyte accumulation, usually in the alveolar spaces of the peribronchial regions with interstitial fibrosis, was observed in many of the specimens.
The histologic patterns of alveolar damage were observed across the full spectrum of diseases ranging from the early exudative/inflammatory phase to the extensive fibroproliferative/fibrosing phase. Early lesions were characterized by bronchiolar and peribronchiolar parenchymal inflammatory infiltration with eosinophilic edema in the alveoli (Fig. 1A). Bronchiolar epithelia was denuded or replaced by flattened epithelia. Mild subepithelial fibroblastic proliferation was also observed (Fig. 1B). The alveolar septal architecture was relatively preserved, although varying degrees of thickening due to inflammatory cell infiltration was observed. In addition to edema fluid, some alveoli showed fibrin plugs or hyaline membranes (Fig. 1C) and accumulation of alveolar macrophages. In the late phase, the parenchymal architecture was remodeled by inflammation and fibrosis which predominated in centrilobular area (Fig. 1D). These included septal fibroblastic proliferation and collagen fibrosis, intra-alveolar fibroblastic plugs with mural incorporation, and bronchiolar destruction with scarring (Fig. 1E, F). The ring fibrosis that occurs in end-stage DAD was not observed. Subpleural and paraseptal airspaces were relatively preserved even in end-stage explanted lung. Type II pneumocyte hyperplasia and residual hyaline membranes were identified in some cases. Other histologic findings characteristic of acute lung injury and the subsequent organizing process were also observed.
In the early phase, focal patchy consolidation was predominantly in the lower lung lobes with the presence of subpleural sparing (Fig. 2A, Table 4). With disease progression, diffuse centrilobular ground-glass opacity was observed with a decrease in consolidation. In the late fibrotic phase, diffuse centrilobular nodules with ground-glass opacity were observed, representing diffuse centrilobular fibrosis (Fig. 2B). Honeycomb change was not identified in any case. At the 1-yr follow up, CT scans of the survivors showed decreased density of centrilobular fibrosis (Fig. 2C). The seven non-survivors developed diverse degrees of air leak (pneumothorax, pneumomediastinum, or subcutaneous emphysema) as a result of bronchiolar fibrosis, but the three survivors with air leak resolved with treatment.
All the patients received methylprednisolone or prednisolone (Table 5). The treatment protocol for the 12 patients admitted to the hospital since 2009 involved a combination of steroids, immune globulin, hydroxychloroquine, and cyclophosphamide. The patients were treated with steroid pulse therapy (methylprednisolone, 30 mg/kg/dose for 3 days) followed by intravenous methylprednisolone or oral prednisolone (2-4 mg/kg/day) with subsequent gradual tapering. The treatment for the four patients admitted between 2006 and 2008 was steroid monotherapy in one patient and a combination of steroids, immune globulin, and hydroxychloroquine in three patients. The type of treatment regimen has no significant effect on patient outcome (Table 5).
The overall mortality rate was 44%, with most deaths occurring between 2 to 4 months after the onset of illness. The mean follow-up period after discharge for the nine survivors was 43.6 months (range, 23-76 months). No relapses have occurred to date. Five patients in the survivor group experienced some dyspnea on moderate activity during follow-up, and have gradually improved.
To identify prognostic factors, we defined the poor prognostic group as those patients who died despite active treatment. The age of symptom onset, interval from symptom onset to treatment, treatment regimens, APACHE II score at admission, and features on lung biopsies and chest CT images were not significantly different between the survivors and non-survivors (Table 3). By contrast, pneumothorax, pneumomediastinum, or widespread subcutaneous emphysema on the chest CT images combined with respiratory difficulty requiring mechanical ventilator support were significantly associated with a poor prognosis (P = 0.001). These suggest that air leak combined with severe respiratory difficulty is associated with poor prognosis.
chILD comprises a large heterogeneous group of rare, mostly idiopathic disorders characterized by diffuse infiltrates, restrictive functional defects, and abnormal gas exchange (8). After we encountered the first few chILD patients in 2006, we became alert to patients with similar clinical courses because of its high mortality rate. This study describes the clinical spectrum, pathologic, and radiological features of 16 children (9 boys and 7 girls), including 5 familial cases, with rapid progression of chILD induced by toxic inhalation. The clinical, pathological, and radiological features in this disease entity were similar to those of adult cases (9). At first, we did not focus on the inhalation toxicity as one of the possible causes of this entity of chILD. However, a nationwide retrospective and prospective case-control study showed that there appears to exist an association between the inhalation toxicity of humidifier disinfectants and chILD induced by toxic inhalation (6). For this reason, we only included children with biopsy-proven toxic inhalation lung injury.
Our patients often had a history of prior illness with constitutional symptoms such as cough, tachypnea, or mild fever. Severe dyspnea developed in some patients over several days after the first symptom presentation. In some patients, hypoxemia developed early in the course of the disease and progressed rapidly to respiratory failure.
In all cases, clinical, but not pathologic and radiologic findings were comparable, but different with those of AIP (e-Table 1). Histologically, this disease was characterized by combination of bronchiolar destruction with mild to severe bronchiolar obliteration and centrilobular distribution of alveolar damage and remodeling by inflammatory and fibroblastic proliferation. Chest CT findings were well correlated with pathologic features, which were characterized by subpleural sparing in the early phase and diffuse centrilobular nodules with ground-glass opacity in the late phase. Some histologic features of our cases, such as bronchiolocentric distribution of inflammation and fibrosis with subpleural sparing, are also observed in idiopathic bronchiolocentric interstitial pneumonia/fibrosis (9, 10). However, significant bronchiolar damage in combination with peribronchiolar alveolar parenchymal remodeling distinguishes our cases from idiopathic bronchiolocentric interstitial pneumonia/fibrosis. We concluded that these cases could not be categorized into any of the disease entities as defined by the classification of chILD. Additionally, these typical pathologic features focusing on severe peribronchiolar inflammation and familial outbreaks may prompt researchers to investigate various environmental exposures for future etiologic investigations in Korea.
Nationwide pediatric surveillance studies performed in Korea in 2006 and 2008 demonstrated a geographical and seasonal pattern for chILD induced by toxic inhalation, which developed from the early spring to early summer, and was observed in the southern regions to the northern regions of the country, reflecting the effects of climate change. In addition, several familial clusters of this disease entity were reported (9, 11).
A recent classification of pediatric diffuse lung disease considers the factors of maldevelopment, genetic background, and interactions between ongoing growth and development (12, 13). Infection, either chronic or acute with long-term complications, airborne allergens, environmental toxins, and commercial chemicals account for many cases of ILD of known etiology (14). For all patients, results of tests for vascular and autoimmune diseases and immune deficiencies were negative. Furthermore, most of the patients in this study had nonproductive cough without fever and chills, and infection was not considered a direct cause in the development of the associated lung injury. Indeed, cultures of lung tissue specimen or bronchoalveolar lavage fluid had failed to provide any evidence of viral (Respiratory syncytial virus, adenovirus, influenza virus, parainfluenza virus, cytomegalovirus, Epstein-Barr virus) or bacterial infection in all patients.
Altogether, we found that this disease has common features with the seasonal spring epidemics, such as the characteristic pattern of its spread from South to North of the Korean peninsula, the simultaneous development of the disease in some family members, and most importantly, the pathognomonic pathological features, including bronchiolar destruction and centrilobular distribution of DAD with subpleural sparing. The different types of viruses detected from nasopharyngeal aspirates by reverse transcription polymerase chain reaction in 5 patients (e-Table 2) may have aggravated the disease; however, infectious agents were not considered a direct cause of this disease because of the lack of consistency in the results of virus detection test in our study. As to the cause of this disease entity, exposure to specific environmental factors such as inhaled environmental agents, commercial chemicals, or toxins may be associated with the development of this disease on the basis of clinical and pathologic findings and a case-control study (6). The Korea Center for Disease Control (KCDC) revealed that some chemical components such as polyhexamethylene guanide (PHMG) and oligo (2-[2-ethoxy] ethoxyethyl guanide chloride) (PGH) in humidifier disinfectants cause similar lung injuries in animal studies (15). The use of humidifier disinfectants in winter and early spring might be associated with disease outbreak and pathologic findings, strongly suggesting that toxic inhalation-associated chILD is causally related to the inhalation of some chemicals or toxins associated with humidifier disinfectants (7, 16). Pulmonary inhalational toxicants such as irritant gases, antigens, or sensitizers other than PHMG and PGH might cause acute inhalation injury through tissue asphyxiation by inhibiting mitochondrial electron transport and oxygen use or by direct airway cellular injury (17). We informed the public about the relationship between this disease and humidifier disinfectant, and presently, we performed a prospective nationwide surveillance study. Since the KCDC warning of the dangers of humidifier disinfectant has been released, there have been no new similar cases in Korea. The association between the KCDC announcement and the absence of new occurrences provides strong additional evidence of the cause being humidifier disinfectants.
Although genetic studies were not conducted for any of our cases including familial cases, the simultaneous development of this disease in family members of different ages, the seasonal distribution in disease outbreak, and its rarity of genetic basis of familial interstitial lung disease suggests that the possibility of genetic-based familial interstitial pneumonia is low (18, 19). Although little information is currently available that indicates why specific individuals are susceptible to a given chemical or toxic reaction, we speculate that the amount and duration of exposure to chemicals or toxic agents may affect the severity and prognosis of this disease. With respect to the prevalent usage of humidifier disinfectants, the occurrence of lung injury in specific individuals is more associated with host susceptibility than cumulative exposure. Further studies are required about factors determining the occurrence and/or severity of lung injury in individual subjects, as we cannot exclude the genetic contribution to lung injury in susceptible individuals.
This disease showed a characteristic pattern of chest CT images with patchy consolidation with subpleural sparing in the early phase and diffuse centrilobular nodules with ground-glass opacity in the late phase. A radiological differential diagnosis of rapidly progressive bronchiolitis obliterans interstitial pneumonia depends on the stage of the disease and includes atypical pneumonia, AIP, and hypersensitivity pneumonitis (HP). Although chest CT scans showed patterns similar to those of the acute and severe phases of HP, there were some differences in radiologic features and treatment responses. The radiologic manifestations of HP include acute pulmonary edema in the acute phase, ground-glass opacities, poorly defined small centrilobular nodules, lobular areas with decreased attenuation, air trapping on images during expiration in the subacute phase; and the reticulation and traction bronchiectasis in the chronic phase (20). In addition, patients with HP usually respond well to corticosteroid therapy and have a good prognosis.
Although current treatment strategies for chILD are unsatisfactory, which is reflected in the high morbidity and mortality (21, 22), the importance of early diagnosis and treatment has been emphasized in previous studies (23). In our institution, different treatment protocols were used between 2006 and 2008 for the treatment of unclassified interstitial pneumonia with fibrosis, depending on the severity of the disease (Table 5). In recognition of its rapid progression and high mortality, the treatment regimen since 2009 has comprised a combination of steroids, cyclophosphamide, immune globulin and hydroxychloroquine. There was no significant difference in outcomes between the treatment regimens. We speculate that treatment unresponsiveness may be due to established pulmonary inflammation with fibrosis prior to treatment initiation or to rapid progression of the disease.
There was great variability in the course of this disease and its response to treatment. The relatively high mortality rate of this disease, 44% in this study compared with a reported range of between 12.5% and 100% for AIP (24-26), may be attributable to a number of factors including different responses to treatment, the extent of fibrosis, and the development of air leak as a result of disease progression. The presence of air leak combined with severe respiratory difficulty reflects the disease severity and may predict poor response to treatment and poor prognosis. Moreover, poor prognosis may be related to genetic susceptibility, host responsiveness, and duration and the amount of exposure to unknown environmental chemicals.
The limitations of this study are as follows. First, we did not investigate the mechanisms underlying the association between specific causative chemical components and the development of this disease. A series of case-control study following this article describes the association as one of the possible causes of chILD (6). Secondly, we did not quantify proinfalmmatory and anti-inflammatory markers such as tumor necrosis factor-alpha and interleukin-10 to support evidence of fibrosis or inflammation in lung tissue or serum. However, radiologic examination and pathologic review of lung biopsies revealed pulmonary fibrotic changes. Finally, this study does not cover the entire disease spectrum of humidifier disinfectant-associated lung injuries; therefore additional studies regarding the natural course of this disease and milder cases than that discussed in our report are needed. This study is useful to investigate the characteristics of patients with similar clinical, radiological, and pathologic findings.
In conclusion, this study describes the clinical, radiological, and pathologic features of chILD induced by inhalation toxicity of humidifier disinfectant. It is characterized by nonproductive cough and dyspnea followed by rapidly progressive respiratory failure with pulmonary fibrosis showing with high mortality. Although this fatal disease may not be a new disease entity but a type of pediatric interstitial lung disease with a new lung injury pattern caused by some toxic agents, avoiding toxic agents such as humidifier disinfectants may prevent the further development of this fatal disease entity. Early diagnosis and timely treatment in addition to preventing exposure to risk factors such as potential respiratory chemicals or toxins may improve prognosis. Further clinical and animal studies on this disease entity are required to understand the clinical phenotype and pathogenesis, to identify the causal etiology clearly, and to determine early diagnostic criteria or biomarkers involved in this disease.
Figures and Tables
Fig. 1
Lung histology in two patients with toxic inhalational lung injury associated with interstitial lung disease in children. (A) Air spaces are diffusely filled with edema fluid. Alveolar septa are focally infiltrated by lymphocytes (H&E, original magnification ×200). (B) A few bronchioles are disrupted and infiltrated by lymphocytes (arrows) (H&E, Original magnification ×400). (C) Alveolar septa are thickened by inflammatory infiltration. Hyaline membranes are deposited air-side of alveolar septa (arrow). Histiocytes with occasional foamy change fill alveolar spaces (H&E, original magnification ×400). (D) Low magnification of this example shows prominent centrilobular distribution of interstitial thickening and fibrosis (H&E, Original magnification ×40). (E) Bronchioles are destructed by inflammatory cells (arrow) and fibroblastic proliferation (asterisk) and epithelial cells are denuded. Peribronchiolar interstitial septa are severely thickened with infiltration of chronic inflammatory cells, fibroblasts and foamy histiocytes (left half) (H&E, Original magnification ×200). (F) Fibroblastic proliferation in pale myxoid stroma obliterates the bronchiolar space (asterisk). Collapsed alveolar spaces are lined by activated pneumocytes and filled with collection of foamy histiocytes (arrow) (H&E, original magnification ×200).
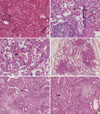
Fig. 2
Radiologic findings in a 41-month-old boy with toxic inhalational lung injury associated interstitial lung disease. (A) A high resolution computed tomography scan performed 2 weeks after symptom onset demonstrates focal patchy consolidation and ground-glass opacity in both lower lobes with subpleural sparing. (B) Chest CT scan of the patient 6 weeks after symptom onset shows progression of diffuse centrilobular nodules with ground-glass opacity, suggesting peribronchiolar fibrosis in both lungs. (C) One-year follow-up chest CT scan shows decreased density, but residual diffuse centrilobular ground-glass opacity involving both lungs.

Table 1
Demographic characteristics of the patients with toxic inhalational lung injury associated with interstitial lung disease

Table 2
Symptoms of the patients with toxic inhalational lung injury associated with interstitial lung disease

Table 3
Clinical characteristics of survivors and non-survivors with toxic inhalational lung injury associated with interstitial lung disease

Table 5
Comparisons of treatment regimens between survivors and nonsurvivors in toxic inhalational lung injury associated interstitial lung disease
ACKNOWLEDGMENTS
We extend our sincere thanks to the children and families who participated in this study.
References
1. Deschildre A, Leclerc F, Hue V, Martinot A, Flurin V, Fourier C, Devisme L, Ramon P, Wallaert B. Treatment with intermittent high dosage corticotherapy in chronic interstitial pneumonia in an infant: a case report. Rev Mal Respir. 1994. 11:509–512.
2. Clement A, Eber E. Interstitial lung diseases in infants and children. Eur Respir J. 2008. 31:658–666.
3. Cheon CK, Jin HS, Kang EK, Kim HB, Kim BJ, Yu J, Park SJ, Hong SJ, Park JD. Epidemic acute interstitial pneumonia in children occurred during the early 2006s. Korean J Pediatr. 2008. 51:383–390.
4. Kim BJ, Kim HA, Song YH, Yu J, Kim S, Park SJ, Kim KW, Kim KE, Kim DS, Park JD, et al. Nationwide surveillance of acute interstitial pneumonia in Korea. Korean J Pediatr. 2009. 52:324–329.
5. Küpeli E, Karnak D, Kayacan O, Beder S. Clues for the differential diagnosis of hypersensitivity pneumonitis as an expectant variant of diffuse parenchymal lung disease. Postgrad Med J. 2004. 80:339–345.
6. Yang HJ, Kim HJ, Yu JH, Lee E, Jung YH, Kim HY, Seo JH, Kwon GY, Park JH, Gwack J, et al. Inhalation toxicity of humidifier disinfectants as a risk factor of children's interstitial lung disease in Korea: a case-control study. PLoS One. 2013. doi: 10.1371/journal.pone.0064430.
7. Kim JY, Kim HH, Cho KH. Acute cardiovascular toxicity of sterilizers, PHMG, and PGH: severe inflammation in human cells and heart failure in zebrafish. Cardiovasc Toxicol. 2013. 13:148–160.
8. Langston C, Fan LL. Diffuse interstitial lung disease in infants. Pediatr Pulmonol. 2001. Suppl 23. 74–76.
9. Lee E, Seo JH, Kim HY, Yu J, Song JW, Park YS, Jang SJ, Do KH, Kwon J, Park SW, et al. Two series of familial cases with unclassified interstitial pneumonia with fibrosis. Allergy Asthma Immunol Res. 2012. 4:240–244.
10. Yousem SA, Dacic S. Idiopathic bronchiolocentric interstitial pneumonia. Mod Pathol. 2002. 15:1148–1153.
11. Kwon SY, Kim JM, Sohn MH, Kim DS, Kim MJ, Cho SH. Acute interstitial pneumonia in siblings: a case report. J Korean Med Sci. 2008. 23:529–532.
12. Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007. 176:1120–1128.
13. Langston C, Dishop MK. Diffuse lung disease in infancy: a proposed classification applied to 259 diagnostic biopsies. Pediatr Dev Pathol. 2009. 12:421–437.
14. Dishop MK. Paediatric interstitial lung disease: classification and definitions. Paediatr Respir Rev. 2011. 12:230–237.
15. Korea Centers for Disease Control and Prevention. accessed on 30 January 2013. Available at http://www.cdc.go.kr/CDC/intro/CdcKrIntro0201.jsp?menuIds=HOME001-MNU0005-MNU0011&cid=9437.
16. Ministry of Health & Welfare. accessed on 30 January 2013. Available at http://www.mw.go.kr/front/mw_sch/index.jsp.
17. Miller K, Chang A. Acute inhalation injury. Emerg Med Clin North Am. 2003. 21:533–557.
18. Amin RS, Wert SE, Baughman RP, Tomashefski JF Jr, Nogee LM, Brody AS, Hull WM, Whitsett JA. Surfactant protein deficiency in familial interstitial lung disease. J Pediatr. 2001. 139:85–92.
19. Nogee LM. Genetics of pediatric interstitial lung disease. Curr Opin Pediatr. 2006. 18:287–292.
20. Silva CI, Churg A, Müller NL. Hypersensitivity pneumonitis: spectrum of high-resolution CT and pathologic findings. AJR Am J Roentgenol. 2007. 188:334–344.
21. King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005. 172:268–279.
22. Fan LL, Langston C. Pediatric interstitial lung disease: children are not small adults. Am J Respir Crit Care Med. 2002. 165:1466–1467.
23. Hewitt CJ, Hull D, Keeling JW. Fibrosing alveolitis in infancy and children. Arch Dis Child. 1977. 52:22–37.
24. Olson J, Colby TV, Elliott CG. Hamman-Rich syndrome revisited. Mayo Clin Proc. 1990. 65:1538–1548.
25. Quefatieh A, Stone CH, DiGiovine B, Toews GB, Hyzy RC. Low hospital mortality in patients with acute interstitial pneumonia. Chest. 2003. 124:554–559.
26. Suh GY, Kang EH, Chung MP, Lee KS, Han J, Kitaichi M, Kwon OJ. Early intervention can improve clinical outcome of acute interstitial pneumonia. Chest. 2006. 129:753–761.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download