Abstract
We attempted to investigate the correlation between the severity of atopic dermatitis (AD) in children and the indoor level of house dust mite (HDM) allergens. Ninety-five patients (31.1 ± 19.5 months of age) with AD were enrolled in this study, and serum specific IgE against Dermatophagoides pteronyssinus and D. farinae was measured. The severity of AD was assessed using the visual analogue scale on the same day of house dust collection. Living rooms and mattresses where the child usually slept were vacuumed for 2 minutes and concentrations of Der f 1 were measured by enzyme-linked immunosorbent assay. The skin symptoms were more severe in patients with Der f 1 concentrations in living room > 2 µg/g dust than ≤ 2 µg/g dust (P = 0.018). This difference was noted in AD patients without sensitization to HDM (P = 0.004), but not in patients with sensitization. There was no difference in symptom severity according to Der f 1 concentrations in mattresses (P = 0.062). The severity of skin symptoms is associated with indoor concentrations of HDM in children with AD, and it is likely to act as nonspecific irritants as well as allergens in AD skin lesions.
Dermatophagoides pteronyssinus and D. farinae are common inhabitants in homes in temperate climates and are major contributors to the allergen concentrations of house dust (1). Previous reports have demonstrated that about 35% of patients with allergic diseases are sensitized to house dust mites (HDM) (2). It is well established that exposure to HDM is associated with development of allergic rhinitis or asthma in children (3, 4), and removal of HDM has been suggested to improve bronchial hyperresponsiveness in asthmatic patients (5).
Atopic dermatitis (AD) is a chronic and highly pruritic inflammatory skin disease with a prevalence of 12.8%-26.5% in children (6, 7). Previous studies have attempted to document the relationship between indoor HDM levels and the development of AD (8, 9), but there has been relatively little information in the literature regarding the effect of HDM concentrations on skin symptoms in patients with AD. Moreover, there are controversies about the relationship between HDM and AD, whereas asthma or allergic rhinitis shows a strong relationship with exposure to HDM (4, 5). For example, it has been demonstrated that the skin and homes of patients with eczema have higher concentrations of mites than those of healthy people, and consequently, reduction of exposure to HDM may result in clinical improvement of eczema (9, 10). On the other hand, it has been reported that domestic HDM exposure was not correlated with SCORing of AD (SCORAD), and no improvement of disease activity was observed in adult patients with AD undertaking 1 yr of HDM avoidance measures (11, 12).
A better understanding of the relationship between AD and HDM exposure in areas where exposure to HDM is ubiquitous may help us to prevent aggravation of skin symptoms in patients with eczema. This is especially relevant for children with AD, since AD requires a comprehensive long-term strategy in the setting of limited therapeutic options (13). Therefore, we attempted to investigate the relationship between the severity of AD in children and the indoor level of HDM allergens in this study.
Ninety-five patients (median age: 23.0 months; range: 2-168 months) with AD as defined by the criteria of Hanifin and Rajka (14) were included in this study. None of the patients had received systemic corticosteroids during the 2 months prior to the clinical evaluation. During the study period, all of the patients were asked to take a bath once daily with warm water for 5 to 10 min and apply moisturizers frequently. Intermittent use of low potency topical corticosteroids (TCS) was allowed in patients who present with erythema and itching. For the patients requiring TCS as rescue medicine, we offered prednisolone valeroacetate or 1% hydrocortisone, and educated the patients to cover the body area equivalent to 2 hands using one fingertip unit of TCS.
The severity of AD was evaluated by the use of the visual analogue scale (VAS) (15). Parents were asked to quantify the overall AD symptoms on a VAS ranging from 0 (no symptoms at all) to 10 (very severe symptoms) on the day of house dust collection. The answer was recorded to E-VAS in response to the question, "How was the eczema in the last month?"; I-VAS to "How were itching symptoms in the last month?"; and S-VAS to "How were sleep-disturbing symptoms in the last month?" E-VAS, I-VAS, and S-VAS were added up to produce T-VAS (VAS of 0-30).
The use of medications was recorded as rescue medicine consumption index (RMCI) to compare their treatment during the last 1 month (15). Allowed medications for AD were for short courses (3 days) of TCS and/or oral hydroxyzine on demand in the case of worsening pruritus, itching, edema, or oozing. When the bacterial infection was suspected, the patients were prescribed a 7-day course of 1st generation cephalosporin. The use of medications was scored 1 point for each dose of oral hydroxizine or topical prednosolone valeroacetate ointment and 2 points for each dose of oral antibiotics over the 7-day course.
Blood samples were collected for measurement of the total IgE level and specific IgE (sIgE) level at the initial visit. The sIgE antibodies to D. pteronyssinus, D. farinae, and common food allergens including egg white, cow's milk, wheat, soy, peanut, and buckwheat were measured by an ImmunoCAP system (Thermo Fisher Scientific Inc., Waltham, MA, USA), with concentrations ≥ 0.35 kU/L being regarded as sensitization.
Dust samples were taken from living rooms and mattresses. A standard household vacuum cleaner (Majestic 360, 1050 W; HMI Industries, Strongsville, OH, USA) equipped with 10 µm filter paper was used. Living room and mattress samples were standardized by vacuuming an area of 1 × 1 m for 2 min. Exposed filter papers were stored at -20℃ until allergen analysis. Each sample was sieved through a 355 nm mesh sieve, recording weights before and after sieving, and 2.0 mL PBS-T (0.05% Tween 20 in phosphate buffered saline, pH 7.4) was added to 100 mg of sieved dust. A proportionate amount of buffer was added if the weight of the dust was between 30 and 100 mg. The dust samples were constantly rotated at room temperature for 2 hr and the aqueous layer was removed after centrifugation at 2,500 rpm at 4℃ for 20 min and stored at -20℃. Der f 1 concentrations were determined using monoclonal antibody (mAb) enzyme-linked immunosorbent assay (Indoor Biotechnologies, Cardiff, UK) as described previously (16). The lower limit of detection for the allergen assays was 0.1 µg/g dust, with no upper limit.
The data were analyzed using SAS version 9.1.3 (SAS, Cary, NC, USA). The concentration of Der f 1 was divided into two groups with a reference concentration of 2 µg/g dust, which is a known cutoff value for HDM sensitization (17). Differences in VAS according to Der f 1 concentrations in each location among the AD patients were analyzed using the Wilcoxon two-sample test. AD patients were classified as being in the sensitized group or not according to the result of sIgE antibodies to D. pteronyssinus or D. farinae. Differences in VAS according to Der f 1 concentrations in each group were also analyzed. In addition, patients were divided into 2 age groups; less than 24 months and 24 months or older. Because the use of separate univariate t tests leads to an inflated type 1 error, the Bonferroni's correction was applied to the subgroup analysis by adjusting the P value. A P value < 0.05 was considered to be significant.
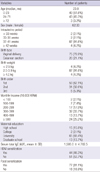
AD patients comprised 62 boys and 33 girls. Their clinical and demographic characteristics were shown in Table 1. Sensitization to HDM was found in 44 out of 95 patients with AD (Table 1). The mean VAS was 5.4 in eczema, 5.3 in itching symptom, 4.1 in sleep-disturbing symptom, and 20.1 in total.
RMCIs were 17.0 ± 16.6 in patients with high (> 2 µg/g dust) Der f 1 levels from living rooms and 13.3 ± 14.4 in those with low (≤ 2 µg/g dust) Der f 1 levels. In addition, RMCIs were 14.7 ± 16.2 in patients with high Der f 1 levels from mattresses and 13.6 ± 14.1 in those with low Der f 1 levels. There were no differences of RMCI between 2 groups with high and low Der f 1 levels from living rooms and mattresses (P = 0.418 and 0.788).
Der f 1 allergen levels were detectable in 24 (25.3%) of 95 samples from living rooms and 79 (83.2%) of 95 samples from mattresses. The highest level was found from mattresses, and Der f 1 levels from mattresses were higher than those from living rooms without statistical significance (P = 0.082). Mean values for Der f 1 (± SD) were 1.4 ± 5.0 (range, 0-33.7) µg/g dust from living rooms and 5.1 ± 19.7 (range, 0-187.0) µg/g dust from mattresses, respectively.
VAS was higher in patients with Der f 1 concentration from living rooms > 2 µg/g dust than in patients with Der f 1 concentration ≤ 2 µg/g dust (P = 0.018) (Fig. 1). In particular, the difference of VAS according to Der f 1 from living rooms was also found in AD patients without sensitization to HDM (P = 0.004). However, the difference of VAS according to Der f 1 from living rooms was not found in AD patients who were sensitized to HDM (P = 1.000). In addition, there were no differences in VAS according to Der f 1 levels from living rooms in younger (<24 months old) age group and older (≥24 months old) age group (P = 0.069 and 0.306).
We did not find differences in VAS according to concentrations of Der f 1 (P = 0.062) from mattresses (Fig. 1). No significant differences in the VAS were apparent between high and low concentrations of Der f 1 from mattresses in AD patients not sensitized to HDM (P = 0.126). There was also no significant difference in the VAS according to concentrations of Der f 1 from mattresses in AD patients sensitized to HDM (P = 1.000). In younger age group, VAS was higher in patients with Der f 1 level from mattresses >2 µg/g dust than in those with Der f 1 level ≤2 µg/g dust (P = 0.007). However, older age group did not show any significant difference in VAS between high and low Der f 1 concentrations from mattresses (P = 1.000)
Our results in this study indicate that indoor HDM levels are associated with the severity of skin symptoms, especially in AD patients without sensitization to HDM. This evidence supports the assertion that we need to reduce the level of HDM to prevent exacerbations of AD. In addition, our findings suggest the possibility that Der f 1 could act as a nonspecific irritant as well as an allergen. The effect of HDM on skin symptoms of AD was not dependent on HDM sensitization, showing their obvious relationship in the non-sensitized group in the present study. These results support the finding that repeated application of D. farinae extract to the skin causes clinical and histological symptoms similar to those in human AD (18). Similarly, in a subgroup of patients with eczema, epicutaneous challenge with HDM extracts resulted in a delayed-type response that resembled naturally-occurring eczema (19). This atopy patch test reaction is regarded as a proteolytic irritancy as well as an IgE-mediated response (9, 19).
Dominant allergens of HDM such as group 1 cysteine proteases, trypsin, chymotrypsin, and a serine protease, possess peptidase activity and have direct effects on mucosal permeability and epithelial function (9, 20). A recent study reported that HDM and cockroach allergens with protease activity can influence homeostasis of the epidermal permeability barrier through protease-activated receptor 2 activation and consequent modulation of the calcium ions in the skin (21). A disruption of the permeability barrier in AD lesions can lead to an increased penetration of environmental allergens into the skin, which initiates immunologic reactions (21). In particular, the disruption of the skin barrier by other stimulants such as volatile organic compounds, can enhance the adverse effects of HDM on provocation testing (22), suggesting that barrier dysfunction of AD skin lesion might lead to a direct pathophysiological role of HDM in AD patients. HDM allergens can be danger signals in the skin through nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin-domain containing 3 (NLRP 3), which plays a central role in both innate immunity and inflammation (23). The results obtained in our study along with previous reports indicate that the proteolytic activities of HDM allergens might play a role as a nonspecific irritant in AD patients.
Although there was no association between indoor HDM level and AD severity in those who were sensitized to HDM, it does not mean that HDM is not an aggravating factor in these patients. IgE-mediated response can be shown even at low allergen levels in selected patients with sensitization. For example, an environmental challenge study demonstrated that similar pulmonary responses between low- and high-allergen conditions in subjects with documented IgE-mediated allergic reactions to rats (24). A previous study showing clinical effect of HDM immunotherapy indicates that HDM can act as an aggravating factor of AD through IgE-mediated mechanism (25). Therefore, in AD patients, regardless of sensitization, HDM is an important aggravating factor which should be reduced in indoor environment.
D. farinae often predominates in Korean homes, although slight variation of mite species has been documented depending on each region (26). Previous studies conducted in the United States also revealed a variable prevalence of mite species between different locations in the same country (27). Few homes showed detectable levels of Der p 1 with a Der f 1/Der p 1 ratio of 11.8 in our preliminary data, which is in agreement with those found in Seoul (Der f 1/Der p 1 ratios of 3.7-27) (26, 28). Preliminary data also showed no significant differences of VAS between high and low Der p 1 levels from living rooms and mattresses (data not shown). For this reason, we used the indoor concentration of Der f 1 not Der p 1 for analyses in this study.
Our study has limitations, mostly stemming from the measurement of HDM. We collected house dust for HDM allergen analysis at only one point of time in various seasons. However, previous studies have reported that Der p 1 and Der f 1 concentrations in children's mattress dust are highly stable, indicating that a single measurement of HDM levels in house dust could be a good indication of exposure to HDM during a 1-yr period (29, 30). We also tried to overcome this limitation by evaluating the severity on the same day of the dust collection, not on the day of subject enrollment. In addition, we evaluated AD severity using VAS, not objective scales such as SCORAD or EASI (eczema area and severity index), because clinicians were not accompanied on the day of measurement. Another potential problem in our study was the use of anti-inflammatory treatment and antihistamine which affect skin lesions and pruritus. However, we compared their medication use through RMCI and found no differences between the two groups.
In conclusion, the severity of skin symptoms is associated with indoor HDM levels in children with AD. These findings are independent of sensitization to HDM, suggesting that, along with the allergenic effects, HDM can act as nonspecific irritants in skin lesions of AD patients. Avoidance of exposure to HDM would be important to prevent exacerbations of skin symptoms in AD, regardless of sensitization to HDM.
Figures and Tables
ACKNOWLEDGMENTS
We thank to Jong Mok Ha for laboratory assistance. The authors have no conflicts of interest to disclose.
References
1. Voorhorst R, Spieksma-Boezeman MI, Spieksma FT. Is a mite (Dermatophagoides sp.) the producer of the house-dust allergen? Allerg Asthma (Leipz). 1964. 10:329–334.
2. Kim KH, Kim KT, Lee SK, Park HS, Lee YM, Nahm DH, Son CH, Yang DK, Roh MS, Choi PJ, et al. Sensitization rates for inhalant allergens in patients with respiratory allergy in Busan. Korean J Asthma Allergy Clin Immunol. 2005. 25:59–63.
3. Kuehr J, Frischer T, Meinert R, Barth R, Schraub S, Urbanek R, Karmaus W, Forster J. Sensitization to mite allergens is a risk factor for early and late onset of asthma and for persistence of asthmatic signs in children. J Allergy Clin Immunol. 1995. 95:655–662.
4. Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007. 119:307–313.
5. Htut T, Higenbottam TW, Gill GW, Darwin R, Anderson PB, Syed N. Eradication of house dust mite from homes of atopic asthmatic subjects: a double-blind trial. J Allergy Clin Immunol. 2001. 107:55–60.
6. Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, Lee SI. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012. 27:681–685.
7. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, Son JA, Lee SY, Lee SI. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004. 19:716–723.
8. Colloff MJ. Exposure to house dust mites in homes of people with atopic dermatitis. Br J Dermatol. 1992. 127:322–327.
9. Kramer U, Lemmen C, Bartusel E, Link E, Ring J, Behrendt H. Current eczema in children is related to Der f 1 exposure but not to Der p 1 exposure. Br J Dermatol. 2006. 154:99–105.
10. Teplitsky V, Mumcuoglu KY, Babai I, Dalal I, Cohen R, Tanay A. House dust mites on skin, clothes, and bedding of atopic dermatitis patients. Int J Dermatol. 2008. 47:790–795.
11. Gutgesell C, Heise S, Seubert S, Seubert A, Domhof S, Brunner E, Neumann C. Double-blind placebo-controlled house dust mite control measures in adult patients with atopic dermatitis. Br J Dermatol. 2001. 145:70–74.
12. Gutgesell C, Seubert A, Junghans V, Neumann C. Inverse correlation of domestic exposure to Dermatophagoides pteronyssinus antigen patch test reactivity in patients with atopic dermatitis. Clin Exp Allergy. 1999. 29:920–925.
13. Krakowski AC, Eichenfield LF, Dohil MA. Management of atopic dermatitis in the pediatric population. Pediatrics. 2008. 122:812–824.
14. Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 92:44–47.
15. Pajno GB, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, Canonica GW, Passalacqua G. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2007. 120:164–170.
16. Zock JP, Heinrich J, Jarvis D, Verlato G, Norback D, Plana E, Sunyer J, Chinn S, Olivieri M, Soon A, et al. Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006. 118:682–690.
17. Kuehr J, Frischer T, Meinert R, Barth R, Forster J, Schraub S, Urbanek R, Karmaus W. Mite allergen exposure is a risk for the incidence of specific sensitization. J Allergy Clin Immunol. 1994. 94:44–52.
18. Yamamoto M, Haruna T, Yasui K, Takahashi H, Iduhara M, Takaki S, Deguchi M, Arimura A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol Int. 2007. 56:139–148.
19. Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wuthrich B, Borelli S Jr, Giusti F, Seidenari S, Drzimalla K, et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. 2004. 59:1318–1325.
20. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999. 104:123–133.
21. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008. 128:1930–1939.
22. Huss-Marp J, Eberlein-Konig B, Breuer K, Mair S, Ansel A, Darsow U, Kramer U, Mayer E, Ring J, Behrendt H. Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy. 2006. 36:338–345.
23. Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, Yang L, Hirakawa S, Hashimoto K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J Allergy Clin Immunol. 2011. 127:806–814.e1-4.
24. Eggleston PA, Ansari AA, Adkinson NF Jr, Wood RA. Environmental challenge studies in laboratory animal allergy. Effect of different airborne allergen concentrations. Am J Respir Crit Care Med. 1995. 151:640–646.
25. Bussmann C, Maintz L, Hart J, Allam JP, Vrtala S, Chen KW, Bieber T, Thomas WR, Valenta R, Zuberbier T, et al. Clinical improvement and immunological changes in atopic dermatitis patients undergoing subcutaneous immunotherapy with a house dust mite allergoid: a pilot study. Clin Exp Allergy. 2007. 37:1277–1285.
26. Ree HI, Jeon SH, Lee IY, Hong CS, Lee DK. Fauna and geographical distribution of house dust mites in Korea. Korean J Parasitol. 1997. 35:9–17.
27. Arlian LG, Bernstein D, Bernstein IL, Friedman S, Grant A, Lieberman P, Lopez M, Metzger J, Platts-Mills T, Schatz M, et al. Prevalence of dust mites in the homes of people with asthma living in eight different geographic areas of the United States. J Allergy Clin Immunol. 1992. 90:292–300.
28. Choi JY, Sohn MH, Jang GC, Lee KE, Kim KE. Concentrations of dust mite in the dust of childhood bedclothing, cloth wrappers, and sewing dolls. Pediatr Allergy Respir Dis. 2002. 12:185–191.
29. Heinrich J, Hölscher B, Douwes J, Richter K, Koch A, Bischof W, Fahlbusch B, Kinne RW, Wichmann HE. Reproducibility of allergen, endotoxin and fungi measurements in the indoor environment. J Expo Anal Environ Epidemiol. 2003. 13:152–160.
30. Gent JF, Belanger K, Triche EW, Bracken MB, Beckett WS, Leaderer BP. Association of pediatric asthma severity with exposure to common household dust allergens. Environ Res. 2009. 109:768–774.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download