Abstract
Polyunsaturated fatty acids (PUFAs) are known to play important roles in various physiological and pathological processes. Recent studies have shown that some omega-3 (ω-3) PUFAs, such as eicosapentaenoic acid (EPA) and dodecahexaenoic acid (DHA), have protective effects on acute and chronic UV-induced changes. However, the effects of other ω-3 PUFAs including 11,14,17-eicosatrienoic acid (20:3) (ETA) on UV-induced skin damages are poorly understood. In this study, we investigated the cutaneous photoprotective effects of ETA in hairless mice in vivo. Female HR-1 hairless mice were topically treated with vehicle (ethanol:polyethylene glycol=30:70) only, 0.1% ETA, or 1% ETA once a day for 3 successive days after one time UV irradiation (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 hr after UV irradiation). We found that topical treatment with ETA attenuated UV-induced epidermal and dermal thickness and infiltration of inflammatory cells, and impairment of skin barrier function. In addition, ETA suppressed the expression of IL-1β, COX-2, and MMP-13 induced by UV irradiation. Our results show that the topical application of ETA protects against UV-induced skin damage in hairless mice and suggest that ETA can be a potential agent for preventing and/or treating UV-induced inflammation and photoaging.
Acute or repetitive exposure of skin to UV radiation is known to induce a variety of harmful effects including inflammation, impaired barrier function, premature skin aging and carcinogenesis (1-4). UV-induced skin inflammation is characterized by erythema, edema, and immunosuppression (5, 6). Erythema is known to be primarily mediated by prostaglandin E2 (PGE2). UV-induced PGE2 production in the skin is due to the increased expression of COX-2 (7, 8).
Skin aging can be divided into intrinsic aging and extrinsic aging. Intrinsic aging (also referred to as chronological aging) occurs with increasing age and is strongly associated with genetic factors. Extrinsic aging is mainly caused by repeated exposure to sunlight and therefore also referred to as photoaging. Whereas naturally aged skin is pale and smoothly wrinkled, photoaged skin is roughly wrinkled and associated with dyspigmentation and telangiectasia (9). Skin connective tissue contains several types of extracellular matrix proteins including collagen, elastin, fibronectin, and proteoglycan, among which collagen is the most abundant. The tight control of synthesis and degradation of extracellular matrix components is critical for maintaining a normal connective tissue structure. Alterations in connective tissue structure are also known to be observed in both chronological aging and photoaging (9, 10).
Matrix metalloproteinases (MMPs) are a family of structurally related endopeptidases. MMPs can degrade various extracellular matrix components and are known to play important roles in tissue remodeling during developmental morphogenesis and angiogenesis, tissue repair, arthritis, skin aging, and tumor invasion (11, 12). MMPs can be classified into several subgroups such as collagenases, gelatinases, stromelysins, membrane-type MMPs, and other MMPs, according to their structures and substrate specificities (13, 14). MMP expression is usually low in unstimulated skin cells or normal skin tissues, but the expression of some MMPs is induced by various extracellular stimuli, such as ultraviolet or infrared radiation, growth factors, cytokines, and tumor promoters (2, 13, 14). Recent studies have shown that hairless mice chronically exposed to UV and/or infrared radiation show epidermal hyperplasia, and skin wrinkles, and significant increase of several MMPs including MMP-13, MMP-2, MMP-9, and MMP-3 (15, 16). These results suggest that MMPs are directly involved in the skin photoaging process, and that the inhibition of the activities of MMPs (either directly by a specific inhibitor or indirectly by reducing their expression) may provide an effective therapeutic method of counteracting photoaging.
Essential fatty acids can be divided into two principal families depending on their saturation state. Omega-3 (ω-3) and omega-6 (ω-6) polyunsaturated fatty acids (PUFAs) have an unsaturated carbon at the third carbon and the sixth carbon in the methyl terminus, respectively. The ω-3 PUFAs include α-linolenic acid (18:3), stearidonic acid (18:4), 11,14,17-eicosatrienoic acid (ETA, 20:3), eicosatetraenoic acid (20:4), eicosapentaenoic acid (EPA, 20:5), docosapentaenoic acid (22:5), and docosahexaenoic acid (DHA, 22:6). The ω-6 PUFAs include linoleic acid (LA, 18:2), γ-linolenic acid (18:3), and arachidonic acid (AA, 20:4). Prostaglandin Es (PGEs) are known to be derived from membrane PUFAs and to play critical roles in carcinogenesis, inflammation, or immune reactions (17, 18). Recent studies have shown that EPA or DHA suppress basal and UV-induced expression of several proinflammatory cytokines and MMPs in skin cells in vitro or skin tissues in vivo (19-21). However, much more work need to be done to fully understand the effects of other members of PUFAs as well as EPA and DHA on UV-induced changes. In this study, we investigated the effect of ETA on UV-induced skin damages in hairless mice in vivo. We found that topical application of ETA significantly prevented clinical changes and molecular events induced by acute exposure of hairless mouse skin to UV radiation.
Six-week-old female albino hairless mice were acclimated for 1 week prior to study and had free access to food and water. Eight mice were allocated to each group (total six groups for each test compounds). All experimental protocols were approved by IACUC (#07-0235) of Clinical Research Institute, Seoul National University Hospital (AAALAC accredited facility).
The UV irradiation device that includes TL20W/12RS UV lamp, with an emission spectrum between 275 and 380 nm (peak, 310-315 nm) was used as the UV source. A Kodacel filter (TA401/407; Kodak, Rochester, NY, USA) was mounted 2 cm in front of the UV lamps to remove UVC wavelengths ≤290 nm. Irradiation intensity on mouse dorsal skin was measured using a UV meter (Model 585100; Waldmann Co., Villingen-Schwenningen, Germany). The irradiation intensity 30 cm from the light source was 1.0 mW/cm2. Initially, we determined the minimal erythemal dose (MED) of mouse dorsal skin. MED was defined as the minimum amount of radiation exposure required to produce erythema with sharp margins after 24 hr. The UV irradiation dose is 200 mJ/cm2 for one time. The vehicle (30% ethanol, 70% polyethylene glycol) only or vehicle containing ETA (Cayman, Ann Arbor, MI, USA) at the final concentration of 0.1% (0.1% ETA) or 1% (1% ETA) was topically applied to the dorsal area (50 µL) after exposure to UV irradiation.
Mouse skin samples were fixed in 10% formalin for 24 hr before processing into paraffin wax. After processing, the samples were embedded in paraffin and 4 µm sections were cut. Serial sections were mounted onto silane-coated slides (Dako, Glostrup, Denmark). Hematoxylin and eosin (H&E) staining was performed and the epidermal thickness and dermal thickness were measured using an image-analyzer (IMTechnology, Daejeon, Korea).
To measure stratum corneum hydration, we used a Corneometer (Courage and Khazaka, Köln, Germany). The Corneometer measures the electrical capacitance at the skin surface, an indicator of skin hydration. Its operation is based on the high dielectric constant of water relative to other skin components.
To measure TEWL, we used a Tewameter (Courage and Khazaka, Köln, Germany). The Tewameter device, a cylindrical probe consisting of two hydrosensors, is designed to measure the rate of water evaporation from the skin surface. TEWL was calculated from the slope provided by the hydrosensors and was computed by averaging the data recorded every 2 sec.
Total RNA was prepared from skin sample using the Trizol method (Invitrogen, Carlsbad, CA, USA) and 1 µg of total RNA was converted to cDNA using First Strand cDNA Synthesis Kit (MBI Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions. To quantitatively estimate the mRNA expression of IL-1β, COX-2 and MMP-13, PCR was performed on a 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA) using the SYBR Premix Ex Taq™ (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. PCR was done using the following primers for the mouse: IL-1β (forward 5'-GAC TCA TGG GAT GAT GAT GAT AAC-3'; reverse 5'-CCA TAC TTT AGG AAG ACA CGG ATT-3'); COX-2 (forward 5'-ATG GAT CGA AGA CTA CGT GCA A-3'; reverse 5'-GGG ATT TCC CAT AAG TCC TTT C-3'); MMP-13 (forward 5'-CAT CCA TCC CGT GAC CTT AT-3'; reverse 5'-GCA TGA CTC TCA CAA TGC GA-3'); 36B4 (5'-TGG GCT CCA AGC AGA TGC-3'; reverse 5'-GGC TTC GCT GGC TCC CAC-3'). The PCR conditions were 50℃ for 2 min, 95℃ for 2 min, followed by 40 cycles at 95℃ for 15 sec and 60℃ for 1 min. Data were analyzed using the 2(-Delta Delta C[T]) method. Data were presented as the fold change in gene expression normalized to 36B4 and relative to the control vehicle group or UV-irradiated vehicle group. These experiments were carried out in triplicate and independently repeated at least three times.
To prepare total skin lysates, skin tissues were homogenized in ice-cold lysis buffer (20 mM Tris-HCL pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA and 1% Triton) with freshly added 5 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM dithiothreitol (DTT) and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Homogenates were then centrifuged at 15,000 g for 30 min at 4℃ and supernatants were collected and aliquots stored at -70℃. Protein contents in lysates were determined using the Bradford assay. Equal amounts (20 µg) of protein were fractionated by 10% SDS-PAGE and transferred to PVDF membranes (Amersham Biosciences, Buckinghamshire, England). Blots were subsequently blocked with blocking buffer (5% nonfat dry milk, 1% Tween-20; in 20 mM TBS, pH 7.6) for 1 hr at room temperature and incubated with anti-COX-2 antibody (Thermo Scientific, Fremont, CA, USA), anti-MMP-13 antibody (Thermo Scientific), anti-IκB antibody (Cell Signaling Technology, Boston, MA, USA), or anti-phospho-NF-κB p65 (Ser 536) antibody (Cell Signaling Technology), overnight at 4℃. After washing, they were then incubated with secondary antibody horseradish peroxidase (Amersham Biosciences) for 1 hr at room temperature and detection using an ECL system (Amersham Biosciences). As controls, the levels of the corresponding β-actin were determined in the same lysates using the antibodies for β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The signal strengths were quantified using a densitometric program (TINA; Raytest Isotopenme β gerate, Straubenhardt, Germany).
Protein levels of mouse IL-1β in skin lysates, and IL-6 secreted into culture media were measured by ELISA, according to the manufacture's protocol (Endogen, Woburn, MA, USA). Briefly, 96 well plates were coated overnight at room temperature with the appropriate monoclonal anti-mouse IL-1β antibodies (1 µg/mL) in 100 µL/well of coating buffer (PBS, pH 7.4). After the coating had been removed, the plates were blocked for 1 hr at room temperature with 200 µL/well of assay buffer (PBS containing 4% BSA, pH 7.2-7.4) and then washed three times with wash buffer (50 mM Tris-HCl [pH 8.0], 0.2% (v/v) Tween-20). Diluted biotinylated detection antibody (50 µL/well) in assay buffer was then added to each well. Serial dilutions of cytokine standard or the supernatants to be tested (100 µL/well) were then added and incubated for 2 h at room temperature. The plates were then washed three times with wash buffer, diluted horseradish peroxidase-conjugated streptavidin (1:10,000) in assay buffer was added, incubated for 30 min at room temperature, and washed three times with wash buffer. Finally, 100 µL of 3,3',5,5'tetramethylbenzidine (TMB) peroxidase substrate solution (0.42 mM TMB/0.003% H2O2 in 0.11 M sodium acetate buffer [pH 5.5]) was added to each well and incubated for 30 min at room temperature. The reaction was stopped by adding 100 µL/well of 0.18 M H2SO4. Optical density was read at 450 nm using a microtiter plate reader.
Acute or chronic UV irradiation has been shown to increase skin thickness and inflammation (15, 16, 22). Therefore, we observed histological changes first to investigate the effects of ETA on UV-induced skin thickening and inflammation. Female HR-1 hairless mice were topically treated with vehicle (ethanol:polyethylene glycol=30:70) only, 0.1% ETA, or 1% ETA once a day for 3 successive days after one time irradiation of UV (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 h after UV irradiation). Serially sectioned samples were stained with H&E and the thickness of epidermis and dermis was measured as described in the Materials and Methods (Fig. 1A). No significant histological changes were observed by topical application of ETA in the skin of control group (Fig. 1A) and skin thickness was not significantly affected by ETA only without UV irradiation (Fig. 1B, C). However, significant histological changes such as skin thickening were observed in UV-irradiated skin (Fig. 1A). The thickness of both epidermis and dermis was significantly increased by UV irradiation (Fig. 1B, C). The mean thickness of epidermis in UV-irradiated vehicle group was increased to 10.1±0.38 µm (P<0.001 vs. control vehicle group, n=8) and that of dermis in UV-irradiated vehicle group was increased to 53.9±2.09 µm (P<0.001 vs. control vehicle group, n=8). Topical treatment with 1% ETA decreased the UV-induced epidermal thickness to 8.8±0.20 µm (P<0.01 vs. UV-irradiated vehicle group, n=8) and the UV-induced dermal thickness to 47.0±1.52 µm (P<0.05 vs. UV-irradiated vehicle group, n=8) (Fig. 1B, C). In addition, UV-irradiation induced dramatic infiltration of inflammatory cells, but topical application of ETA prevented it significantly (Fig. 1A). Taken together, these results indicate that ETA has photoprotective effects on skin of hairless mice.
We also anticipated whether ETA has other advantages on skin, such as skin barrier function. Stratum corneum hydration and TEWL reflect cutaneous barrier function directly. To study the effects of ETA on cutaneous barrier function, we measured stratum corneum hydration and TEWL in control and UV-irradiated mice, respectively. We confirmed that UV decreased stratum corneum hydration and increased TEWL significantly. Stratum corneum hydration (Fig. 2A) was increased whereas TEWL (Fig. 2B) was decreased by ETA, in a dose-dependent manner, in both control and UV-irradiated groups, respectively. These results suggest that topical treatment with ETA improves skin barrier function in hairless mice in vivo.
To understand the molecular mechanisms of the ETA-mediated inhibition of skin thickening and inflammation in UV-irradiated hairless mice, we investigated the effects of ETA on IL-1β and COX-2 that play important roles in inflammation. We examined whether ETA could affect UV-induced IL-1β expression. The mRNA and protein levels of IL-1β were detected by quantitative real-time PCR (Fig. 3A) and ELISA (Fig. 3B) respectively. Both mRNA and protein levels of IL-1β were increased dramatically by UV and the UV-induced IL-1β mRNA and protein levels were significantly decreased by ETA, in a dose-dependent manner (Fig. 3A, B).
UV induces the expression of COX-2 that increases prostaglandin (PG) from AA. PG is known to play important role in inflammation and MMP expression (22, 23). Therefore, we investigated whether ETA could affect UV-induced COX-2 expression. The mRNA and protein levels of COX-2 were determined by quantitative real-time PCR (Fig. 3C) and Western blot analysis (Fig. 3D), respectively. UV-induced COX-2 expression was dramatically increased at both mRNA and protein levels, while application of ETA significantly inhibited the induction of COX-2 mRNA and protein (Fig. 3C, D). These results show that topical application of ETA may prevent the inflammatory reactions caused by UV irradiation.
Exposure of skin to UV radiation has been shown to induce the expression of various MMPs including MMP-13. The induction of MMPs is suggested to be closely correlated with premature skin aging induced by UV in murine or human skin (9, 10). To determine the effects of ETA on UV-induced MMP-13 expression in hairless mice in vivo, the mRNA levels of MMP-13 were analyzed by quantitative real-time PCR (Fig. 4A). In addition, the expression of MMP-13 protein was analyzed by Western blot analysis (Fig. 4B) and immunohistochemistry (Fig. 4C). The UV-induced MMP-13 mRNA expression was significantly reduced by topical application of 1% ETA (Fig. 4A). The MMP-13 protein levels were also greatly decreased by treatment with 0.1% ETA and 1% ETA (Fig. 4C, D). These results indicate that topical application of ETA suppresses UV-induced MMP-13 expression at the both mRNA and protein levels in hairless mice in vivo.
It has been reported that the transcription factor nuclear factor-κB (NF-κB) plays an important role on expression of IL-1β and COX-2 induced by various stimuli (24). In unstimulated cells, NF-κB is present in the cytoplasm as complexes with one of three isoforms (α, β, or ε) of inhibitor of κB (IκB). Upon stimulation with extracellular stimuli including proinflammatory cytokines or UV, the inhibitor of κB kinase (IKK) phosphorylates IκB proteins, targeting them for proteolysis, which leads to translocation of NF-κB into the nucleus where NF-κB induces transcriptional activation of a variety of genes, including proinflammatory cytokines, chemokines, COX-2, and MMPs (25). In addition, NF-κB activity has been shown to be regulated by the phosphorylation of NF-κB p65 (26, 27). To study how ETA affects the molecular events involved in the regulation of NF-κB activity by UV in hairless mice in vivo, we investigated the effects of ETA on UV-induced regulation of IκBα expression and NF-κB p65 phosphorylation. Topical treatment of ETA increased IκBα protein levels in both control group and UV-irradiated group (Fig. 5A). Basal levels of phospho-NF-κB p65 were not affected by ETA but UV-induced levels of phospho-NF-κB p65 were decreased dose-dependently by treatment with ETA (Fig. 5B), indicating that ETA can attenuate UV-induced NF-κB activation. These results suggest that ETA may inhibit UV-induced expression of IL-1β, COX-2 and MMP-13 through the regulation of NF-κB signaling pathways.
Acute or chronic UV irradiation causes various harmful effects including inflammation, premature aging, and carcinogenesis in murine or human skin. The UV-induced damages, such as inflammation and premature aging, are often determined by skin thickness, infiltration of inflammatory cells, skin barrier function, or extracellular matrix remodeling (1, 5, 9). It has been reported that many of UV-induced changes are mediated through the induction of proinflammatory cytokines, chemokines, COX-2, or MMPs (1, 7, 10, 11).
In this study, we investigated the effect of 11, 14, 17-ETA, a ω-3 PUFA, on UV-induced skin damages in hairless mice in vivo. First of all, we analyzed the UV-visible absorption spectrum of ETA dissolved in methanol and found that ETA showed little absorption within the wavelength interval of UV light (data not shown). These results rule out the possibility of physical absorption of UV light by ETA itself. We found that topical application of ETA significantly attenuated UV-induced skin thickening and infiltration of inflammatory cells. In addition, we found that ETA treatment increased the stratum corneum hydration and decreased the TEWL in both control and UV-irradiated mice, in a dose-dependent manner, indicating that ETA can improve skin barrier function. Furthermore, UV-induced expressions of IL-1β, COX-2, and MMP-13 were greatly suppressed at the mRNA and protein levels by topical application of ETA in hairless mouse skin. It has been reported that other ω-3 PUFAs, such as EPA and DHA, suppress the expression of TNF-α, IL-1α, IL-6 or IL-8 and the production of PGE2 in UV-irradiated keratinocytes or fibroblasts (19, 28) and that UV-induced MMP-1 expression is suppressed by treatment with EPA and DHA, ω-3 PUFAs, but not LA and AA, ω-6 PUFAs in human dermal fibroblasts (20). Recent studies have shown that topical application of EPA attenuates the epidermal thickening, the increase of MMP-1, MMP-9, and COX-2 expression, and the decrease of collagen production, induced by UV light in human skin in vivo (21).
Our results showed that ETA could affect UV-induced NF-κB activation through regulation of multiple points in NF-κB signaling pathways. It has been shown that EPA affects many signaling pathways induced by UV or TNF-α in human skin cells in vitro and human skin in vivo (20, 21, 29). Further work needs to be done to elucidate the signaling pathways regulated by ETA.
Taken together, our results showed that ETA, like other ω-3 PUFAs, such as EPA and DHA, had protective effects against UV-induced skin damages and suggested that ETA could be used as a potential therapeutic agent that protects skin against UV-induced inflammation and photoaging.
Figures and Tables
Fig. 1
Topical application of 11,14,17-eicosatrienoic acid (ETA) inhibits UV-induced skin thickening and inflammatory cell infiltration in hairless mouse skin in vivo. Female HR-1 hairless mice were topically treated with vehicle (ethanol:polyethylene glycol=30:70) only, 0.1% ETA or 1% ETA once a day for 3 successive days after one time irradiation of UV (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 hr after UV irradiation). (A) Serial sections were mounted onto silane-coated slides and stained with hematoxylin and eosin (H&E) (magnification ×200). (B, C) Epidermal and dermal thicknesses were measured. Values are mean±SEM (n=8).
*P<0.001 vs. control (CON) vehicle group; †P<0.05; ‡P<0.01 vs. UV-irradiated (UV) vehicle group.
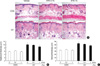
Fig. 2
Topical application of ETA increases stratum corneum hydration and decreases transepidermal water loss (TEWL) in control and UV-irradiated hairless mouse skin in vivo. Female HR-1 hairless mice were topically treated with vehicle (ethanol:polyethylene glycol=30:70) only, 0.1% ETA or 1% ETA once a day for 3 successive days after one time irradiation of UV (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 hr after UV irradiation). (A) Stratum corneum hydration was dramatically decreased after UV irradiation. Stratum corneum hydration was increased by topical application of ETA both in normal and UV-irradiated hairless mice. (B) TEWL was dramatically increased after UV irradiation. Topical application of ETA decreased TEWL both in control and UV-irradiated hairless mice. Values are mean±SEM (n=8).
*P<0.05; ‡P<0.001 vs. control (CON) vehicle group; ‡P<0.05 vs. UV-irradiated (UV) vehicle group.
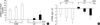
Fig. 3
Topical application of ETA prevents UV-induced interleukin-1beta (IL-1β) and cyclooxygenase-2 (COX-2) expressions in hairless mouse skin in vivo. Female HR-1 hairless mice were topically treated with vehicle (ethanol:polyethylene glycol=30:70) only, 0.1% ETA or 1% ETA once a day for 3 successive days after one time irradiation of UV (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 hr after UV irradiation). (A) Expression of IL-1β mRNA was determined by quantitative real-time RT-PCR. (B) Expression of IL-1β protein was analyzed by ELISA. (C) Expression of COX-2 mRNA was detected by quantitative real-time RT-PCR. (D) Expression of COX-2 protein was detected by Western blot analysis. Results are expressed as fold change. Values are mean±SEM (n=8).
*P<0.01, †P<0.001 vs. control (CON) vehicle group; ‡P<0.05, §P<0.01 ∥P<0.001 vs. UV-irradiated (UV) vehicle group.

Fig. 4
Topical application of ETA prevents UV-induced matrix metalloproteinase-13 (MMP-13) expression. Female HR-1 hairless mice were topically treated with vehicle (ethanol:polyethylene glycol=30:70) only, 0.1% ETA or 1% ETA once a day for 3 successive days after one time irradiation of UV (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 h after UV irradiation). (A) Expression of MMP-13 mRNA was detected by quantitative real-time RT-PCR. (B) Expression of MMP-13 protein was detected by western blot analysis. (C) Immunohistochemical staining of MMP-13 protein (magnification ×200). Results are expressed as fold change. Values are mean±SEM (n=8).
*P<0.001 vs. control (CON) vehicle group; †P<0.05, ‡P<0.01 vs. UV-irradiated (UV) vehicle group.
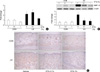
Fig. 5
ETA increases IκBα expression and decreases NF-κB p65 phosphorylation in hairless mouse skin in vivo. Female HR-1 hairless mice were topically treated with vehicle (ethanol : polyethylene glycol=30:70) only, 0.1% ETA or 1% ETA once a day for 3 successive days after one time irradiation of UV (200 mJ/cm2) on dorsal skins. Skin biopsy was carried out on the fourth day (72 h after UV irradiation). (A) Expression of IκBα was detected by western blot analysis. (B) Phospho-NF-κB p65 was detected by western blot analysis. Results are expressed as fold change. Values are mean±SEM (n=8).
*P<0.001 vs. control (CON) vehicle group; †P<0.05, ‡P<0.001 vs. UV-irradiated (UV) vehicle group.

References
1. Kondo S. The roles of cytokines in photoaging. J Dermatol Sci. 2000. 23:Suppl 1. S30–S36.
2. Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002. 1:705–720.
3. Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008. 84:528–536.
4. Halliday GM, Lyons JG. Inflammatory doses of UV may not be necessary for skin carcinogenesis. Photochem Photobiol. 2008. 84:272–283.
5. Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993. 100:35S–41S.
6. Schwarz T. Photoimmunosuppression. Photodermatol Photoimmunol Photomed. 2002. 18:141–145.
7. Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998. 19:723–729.
8. Wilgus TA, Parrett ML, Ross MS, Tober KL, Robertson FM, Oberyszyn TM. Inhibition of ultraviolet light B-induced cutaneous inflammation by a specific cyclooxygenase-2 inhibitor. Adv Exp Med Biol. 2002. 507:85–92.
9. Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989. 21:610–613.
10. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002. 138:1462–1470.
11. Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997. 6:199–213.
12. Woessner JF Jr. Role of matrix proteases in processing enamel proteins. Connect Tissue Res. 1998. 39:69–73.
13. Nagase H, Woessne JF Jr. Matrix metalloproteinases. J Biol Chem. 1999. 274:21491–21494.
14. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001. 17:463–516.
15. Inomata S, Matsunaga Y, Amano S, Takada K, Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y, Fukuda M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 2003. 120:128–134.
16. Kim HH, Lee MJ, Lee SR, Kim KH, Cho KH, Eun HC, Chung JH. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech Ageing Dev. 2005. 126:1170–1177.
17. Herschman HR, Xie W, Reddy S. Inflammation, reproduction, cancer and all that.... The regulation and role of the inducible prostaglandin synthase. Bioessays. 1995. 17:1031–1037.
18. Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003. 100:1751–1756.
19. Storey A, McArdle F, Friedmann PS, Jackson MJ, Rhodes LE. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-alpha-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol. 2005. 124:248–255.
20. Kim HH, Shin CM, Park CH, Kim KH, Cho KH, Eun HC, Chung JH. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J Lipid Res. 2005. 46:1712–1720.
21. Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun HC, Chung JH. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J Lipid Res. 2006. 47:921–930.
22. Tanaka S, Sato T, Akimoto N, Yano M, Ito A. Prevention of UVB-induced photoinflammation and photoaging by a polymethoxy flavonoid, nobiletin, in human keratinocytes in vivo and in vitro. Biochem Pharmacol. 2004. 68:433–439.
23. Mauviel A, Halcin C, Vasiloudes P, Parks WC, Kurkinen M, Uitto J. Uncoordinate regulation of collagenase, stromelysin, and tissue inhibitor of metalloproteinases genes by prostaglandin E2: selective enhancement of collagenase gene expression in human dermal fibroblasts in culture. J Cell Biochem. 1994. 54:465–472.
24. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. 2008. 49:133–143.
25. Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006. 2006:re13.
26. Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004. 279:55633–55643.
27. Schmitz ML, Mattioli I, Buss H, Kracht M. NF-kappaB: a multifaceted transcription factor regulated at several levels. Chembiochem. 2004. 5:1348–1358.
28. Pupe A, Moison R, De Haes P, van Henegouwen GB, Rhodes L, Degreef H, Garmyn M. Eicosapentaenoic acid, a n-3 polyunsaturated fatty acid differentially modulates TNF-alpha, IL-1alpha, IL-6 and PGE2 expression in UVB-irradiated human keratinocytes. J Invest Dermatol. 2002. 118:692–698.
29. Kim HH, Lee Y, Eun HC, Chung JH. Eicosapentaenoic acid inhibits TNF-alpha-induced matrix metalloproteinase-9 expression in human keratinocytes, HaCaT cells. Biochem Biophys Res Commun. 2008. 368:343–349.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download