Abstract
This is a long-term, open label, observational study aimed to broaden our clinical experiences in managing infants and toddlers with epilepsy. The long-term retention rate and side effects of topiramate (TPM) in them were evaluated and compared with carbamazepine (CBZ). A total of 146 children were involved in the study (TPM=41, CBZ=105). The retention rates at 24 , 36, and 48 months were 46.3%, 34.1%, 26.8% for TPM and 36.2%, 23.8%, 13.3% for CBZ, respectively. At 6 months after starting antiepileptic drugs (AED), the seizure freedom or clinical efficacy (seizure reduction rate more than 50 percent) were 73.2% for TPM and 62.9% for CBZ. The major side effects led to discontinuation included psychomotor slowing, poor oral intake from TPM and sleepiness and skin rash from CBZ. TPM was discontinued due to side effects in one case (2.4%) and lack of efficacy in five cases (12.2%), whereas CBZ was discontinued due to lack of efficacy (22.9%) and side effects (6.7%). As compared with CBZ, TPM showed the same long-term retention rate in children under the age of 2 yr, and no serious side effects. It is therefore concluded that TPM can be considered as a major AED for treating children with epilepsy under the age of 2 yr.
Epilepsies in children, especially infants, are a heterogeneous group of conditions that differ from epilepsies in adults not only in the clinical manifestations of seizures but also in etiologies and response to antiepileptic drugs (AEDs). Despite increasing data on treatment of epilepsy, little is known about the use of new AEDs in children especially infants with epilepsies, and many clinical questions need to be answered. Topiramate (TPM), one of new AEDs, was first introduced in the treatment of certain refractory epilepsy syndromes such as Lennox-Gastaut and West syndromes and was believed to be effective for seizure reduction without any serious or life-threatening adverse events in children aged over 4 yr (1-4). However, TPM was associated with a high incidence of side effects in clinical practice, affecting its long-term retention (5).
The aim of this study was to assess the efficacy, tolerability, and safety of TPM in the treatment of infants and toddlers with partial and generalized epilepsies. This study focused on the long-term retention rate and adverse events of TPM compared with carbamazepine (CBZ), a conventional AED.
This is a long-term, open label, observational study. A total of 146 children with epilepsy under the age of 2 yr were involved in the study and were initially prescribed either TPM or CBZ at the pediatric neurology clinic, Kyungpook National University Hospital, Daegu, Korea from January 1, 2000 to December 31, 2003. CBZ was started with 5-10mg/kg/day and increased weekly in increment of 5-10 mg/kg/day to no more than 30 mg/kg/day if necessary. TPM was started at a dose of 0.5-1 mg/kg/day and titrated to 3-9 mg/kg/day by 1 mg/kg/day increment per week. They were on each drug for at least 3 months or longer. The data of the long term retention and side effects of both groups, at 24, 36, and 48 months were assessed. The data for the side effects of both AEDs were collected from their parents' reports and from examiners to minimize the subjective bias. Main reasons for the discontinuation of both AEDs were determined by 3 scales (lack of efficacy, adverse events, and other factors). The data were statistically analyzed using SPSS 12.0. Retention/survival rates were estimated at 24, 36, and 48 months, using parametric Kaplan-Meier and nonparametric Cox proportional regression methods. In analyzing side effects, a chi-square test was performed. The Institutional Review Board for the clinical research formally approved the study (74005-940).
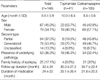
A total of 146 infants and toddlers (male 67, female 79) were enrolled in the study. Their mean age was 8.8±5.9 months old ranging from 1 to 23 months, the mean duration of the follow-up was 32±24 months, and the mean duration of medication was 24±22 months. The mean dosages were 4.9±2.5 mg/kg/day for TPM and 12.8±3.2 mg/kg/day for CBZ. Fifty-four patients (37%) had partial seizures, 78 patients (53.4%) had generalized seizures, and 25 patients (17.1%) had a family history of epilepsy. Fifty-three of the 146 patients had underlying pathology in the brain (Table 1). The clinical characteristics were not statistically different between the two groups. With respect to retention rates, the Kaplan-Meier analysis of the two AEDs revealed estimated retention rates of 46.3% for TPM and 36.2% for CBZ at 24 months, 34.1% (TPM) and 23.8% (CBZ) at 36 months, and 26.8% (TPM), 13.3% and (CBZ) at 48 months (Figs. 1, 2). Log rank testing showed no significant difference between the two AEDs.
Based on the reduction rate of the average monthly seizure frequency, the efficacy was defined by Grade A-F. Grade A was seizure free, Grade B for seizure reduction more than 50%, Grade C for 25-50% of seizure reduction, Grade D for less than 25% of seizure reduction, Grade E for no improvement, and Grade F for when the seizures worsened. At 6 months, Grade A and B patients were 73.1% for the TPM group, and 62.9% for the CBZ group (Fig. 3).
As far as the side effects are concerned, 28 out of the subjects (19.2%) showed side effects which consisted of 24.4% for the TPM group and 17.1% for the CBZ group. No statistically significant difference was noted between the two groups (odd ratio [OR]=1.559, 95% confidence interval [CI]=0.650-3.740).
The most common side effects included sleepiness and psychomotor retardation for the TPM group and sleepiness and rash for the CBZ group. Other side effects are summarized in Fig. 4. The main reasons for discontinuing the drugs were ineffectiveness (TPM; 12.2%, CBZ; 22.9%) and side effects (TPM 2.4%, CBZ; 6.7%) (Fig. 5).
Many studies showed that TPM has a broad spectrum of antiepileptic effects without any potentially dangerous side effects in children (6, 11-16). In contrast to previous studies, we investigated the long term retention rate, the efficacy, and tolerability of TPM compared with CBZ in children with epilepsies, particularly infants in the present study. We admit some limitations and bias such as patient selection, diagnostic tools, choosing the first AED for certain types of seizures, etc. However, this study may provide the clinical evidence in a certain aspect and can be helpful for clinicians.
The data of 146 children under the age of 2 yr with a wide spectrum of epilepsy were reviewed. We focused on the long-term retention rate and adverse events of TPM compared with CBZ. There was no obvious difference between the two groups in demographic and clinical manifestations, but the TPM group had more children with epileptic syndromes or symptomatic epilepsies than the CBZ group (38.5% in CBZ group vs. 53.4% in TPM group).
For evaluating the efficacy, the two groups were observed for seizure freedom or seizure reduction >50% for 6 months or longer. At 6 months after starting the AEDs, about half of patients in both groups became seizure free. At least 50% or more reduction of the average monthly seizure frequency was achieved in 14.6% of the TPM group and 8.6% of the CBZ group respectively. This result is not statistically significant, but we still believe that TPM is more efficacious than CBZ because TPM was given more for the patients with symptomatic epilepsies or epileptic syndromes.
Like other previous studies (17-19), our study also demonstrated that TPM had a similar retention rate over 48 months or longer on survival analysis as compared with CBZ. Since long-term retention rates indicate both effectiveness and tolerability, it may be concluded that TPM is as good as CBZ for treating children under the age of 2 yr. In addition, TPM may have a better efficacy at the expense of some deleterious effects in our study because it was discontinued mainly due to adverse events, not ineffectiveness during the study period, which differs from CBZ.
Based on previous studies, adverse events from TPM include behavioral and cognitive difficulties, anorexia, weight loss, irritability, allergic skin reaction, headaches, paraesthesia and so on. They are usually mild, transient, and often related to rapid titration (7, 8, 20-23). Despite the slow titration, 10 of 41 children (24.4%) in the TPM group suffered from adverse events early during the treatment in this study. When compared with the CBZ group, TPM showed similar side effects, and they were mild and resolved with time or by adjusting dosages. The most common side effects were CNS related symptoms or signs, such as sleepiness and psychomotor retardation. However, this assumption is limited because most of children at this age cannot complain about them and these symptoms can easily be overlooked.
Recent studies reported that anhidrosis was a relatively common side effect of TPM in children and adults (24, 25). In this study, only one of 41 children complained of difficulty with sweating or febrile sensation, which is quite comparable to previous studies. Despite the fact, this side effect can be problematic especially in infants. It therefore should be more cautious when prescribing TPM for an infant in a hot environment.
Monotherapy is known to be effective with minimal side effects (26). In this study, 13 of 41 children in the TPM groups were treated with monotherapy. They achieved seizure freedom without any harmful adverse events. This study suggests TPM monotherapy would be applicable to treat infants or toddlers with a variety of epilepsies.
Finally, there are limited options of AEDs for managing children with refractory epilepsies under the age of 2 yr. TPM can be considered as a treatment option before moving onto surgical or other treatment modalities.
In conclusion, this comparative study had some limitations, but showed the evidence that TPM was as effective as CBZ. TPM did not cause serious side effects in managing children with a variety of different epilepsies under the age of 2 yr. As a result, TPM can be considered as one of major treatment options, preferably monotherapy for treating infants with epilepsies.
Figures and Tables
Fig. 1
Proportion of patients stayed on the initial AED at 24, 36, and 48 months. In the CBZ group, the proportion of patients kept on CBZ was 40.4%, 26.6%, and 14.9% at 24, 36, 48 months, respectively, and 47.7%, 31.8%, and 25.0% in the TPM groups. TPM tends to be continued in higher proportion of patients, but is not statistically different from CBZ.
CBZ, carbamazepine; TPM, topiramate; AED, antipileptic drug.

Fig. 2
Cumulative survival of topiramate (TPM) and carbamazepine (CBZ). The retention rates of TPM and CBZ were compared over 48 months or longer. TPM seemed to have a better retention rate, but was not significantly different from CBZ.

Fig. 3
Comparative efficacy between CBZ and TPM. At 6 months after starting the antiepileptic drug, 58.5% in the TPM group and 54.3% in the CBZ group became seizure free and the average monthly seizure frequency was decreased by 50% or more in 14.6% of the TPM group and 8.6% of the CBZ group respectively. The findings suggest that TPM is as efficacious as CBZ.
Grade A, seizure freedom; Grade B, seizure reduction of average monthly frequency ≥50%; Grade C, seizure reduction of average monthly frequency 25-50%; Grade D, seizure reduction of average monthly frequency ≤25%; Grade E, no improvement in seizures; Grade F, worsening of seizures.
CBZ, carbamazepine; TPM, topiramate; AED, antipileptic drug.

Fig. 4
Adverse events of TPM (A) and CBZ (B). There were 12 events of side effects in 10 of 41 patients treated with TPM. In CBZ treated patients, there were 21 events of side effects in 18 of 105 patients. The most common adverse events were CNS-related in both groups and anhidrosis was another noticeable adverse event in the TPM group.
CBZ, carbamazepine; TPM, topiramate.

Fig. 5
The main reason of discontinuation of AEDs. The leading causes were lack of efficacy (12.2% for TPM, 22.9% for CBZ) and adverse events (2.4% for TPM, 6.7% for CBZ). Others included deaths from acute illnesses, the poor compliance and so on. No statistically significant difference was noted between two groups.
CBZ, carbamazepine; TPM, topiramate; AED, antipileptic drug.

References
1. Aicardi J. Risks and benefits of new antiepileptic agents in children. Rev Neurol. 2000. 31:376–381.
2. Bourgeois BF. Pharmacokinetics and pharmacodynamics of topiramate. J Child Neurol. 2000. 15:Suppl 1. S27–S30.

4. Blumkin L, Lerman-Sagie T, Houri T, Gilad E, Nissenkorn A, Ginsberg M, Watemberg N. Pediatric refractory partial status epilepticus responsive to topiramate. J Child Neurol. 2005. 20:239–241.

5. Bootsma HP, Aldenkamp AP, Diepman L, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M. The effect of antiepileptic drugs on cognition: patient perceived cognitive problems of topiramate versus levetiracetam in clinical practice. Epilepsia. 2006. 47:Suppl 2. 24–27.

6. Wheless JW. Use of topiramate in childhood generalized seizure disorders. J Child Neurol. 2000. 15:Suppl 1. S7–13.

7. Waugh J, Goa KL. Topiramate: as monotherapy in newly diagnosed epilepsy. CNS Drugs. 2003. 17:985–992.
9. Ormrod D, McClellan K. Topiramate: a review of its use in childhood epilepsy. Paediatr Drugs. 2001. 3:293–319.
10. Nicolson A, Appleton RE, Chadwick DW, Smith DF. The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry. 2004. 75:75–79.
11. Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003. 107:165–175.

12. Korinthenberg R, Schreiner A. Topiramate in children with west syndrome: a retrospective multicenter evaluation of 100 patients. J Child Neurol. 2007. 22:302–306.

13. Rosenfeld WE, Doose DR, Walker SA, Baldassarre JS, Reife RA. A study of topiramate pharmacokinetics and tolerability in children with epilepsy. Pediatr Neurol. 1999. 20:339–344.

14. Mikaeloff Y, de Saint-Martin A, Mancini J, Peudenier S, Pedespan JM, Vallee L, Motte J, Bourgeois M, Arzimanoglou A, Dulac O, Chiron C. Topiramate: efficacy and tolerability in children according to epilepsy syndromes. Epilepsy Res. 2003. 53:225–232.

15. Mikaeloff Y, Rey E, Soufflet C, d'Athis P, Echenne B, Vallee L, Bouhours P, Grinspan A, Dulac O, Pons G, Chiron C. Topiramate pharmacokinetics in children with epilepsy aged from 6 months to 4 years. Epilepsia. 2004. 45:1448–1452.

16. Malphrus AD, Wilfong AA. Use of the newer antiepileptic drugs in pediatric epilepsies. Curr Treat Options Neurol. 2007. 9:256–267.

17. Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure. 2007. 16:296–304.

18. Zaccara G, Messori A, Cincotta M, Burchini G. Comparison of the efficacy and tolerability of new antiepileptic drugs: what can we learn from long-term studies? Acta Neurol Scand. 2006. 114:157–168.

19. Bootsma HP, Coolen F, Aldenkamp AP, Arends J, Diepman L, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M. Topiramate in clinical practice: long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2004. 5:380–387.

20. Moreland EC, Griesemer DA, Holden KR. Topiramate for intractable childhood epilepsy. Seizure. 1999. 8:38–40.

21. Mohamed K, Appleton R, Rosenbloom L. Efficacy and tolerability of topiramate in childhood and adolescent epilepsy: a clinical experience. Seizure. 2000. 9:137–141.

22. Voronkova KV, Pylaeva OA, Petrukhin AS. Efficacy of topiramate (Topamax) in epileptic patients of different ages. Neurosci Behav Physiol. 2007. 37:547–551.

23. Nieto-Barrera M, Candau R, Nieto-Jimenez M, Correa A, del Portal LR. Topiramate in the treatment of severe myoclonic epilepsy in infancy. Seizure. 2000. 9:590–594.

24. Zou LP, Lin Q, Qin J, Cai FC, Liu ZS, Mix E. Evaluation of open-label topiramate as primary or adjunctive therapy in infantile spasms. Clin Neuropharmacol. 2008. 31:86–92.

25. Nieto-Barrera M, Nieto-Jimenez M, Candau R, Ruiz del Portal L. Anhidrosis and hyperthermia associated with treatment with topiramate. Rev Neurol. 2002. 34:114–116.
26. Mukhin K, Glukhova L, Petrukhin AS, Mironov MB, Sobornova AM. Topamax in monotherapy of epilepsy. Zh Nevrol Psikhiatr Im S S Korsakova. 2004. 104:35–40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download