Abstract
The adenosine triphosphate-based chemotherapy response assay (ATP-CRA) is a chemosensitivity test that offers the potential of selecting cancer treatments based on the responsiveness of individual tumors. We report a case of 47-yr-old male, presented with sigmoid colon cancer with multiple liver and peritoneal metastases, in which there was a complete response for the primary colon cancer after administration of preoperative chemotherapy selected by ATP-CRA. Oxaliplatin was the most sensitive drug based on the ATP-CRA where the specimen obtained by ultrasound-guided percutaneous liver biopsy was used. After twelve cycles of oxaliplatin-capecitabine chemotherapy, abdominopelvic computed tomography revealed marked shrinkage of the liver metastases and positron emission tomography showed no uptake of 18F-fluoro-deoxy-glucose (FDG) either in the liver or peritoneum except localized uptake in the sigmoid colon. The patient underwent an anterior resection and radiofrequency ablation of the liver metastases, which resulted in a macroscopic curative resection of the cancer cells. Histological examination revealed no residual cancer cells in the resected specimen of the sigmoid colon. This result suggested that preoperative chemotherapy chosen by ATP-CRA may be useful for treating advanced colon cancer with unresectable liver and peritoneal metastases.
Systemic chemotherapy is the treatment of choice in patients with unresectable metastatic colorectal cancer, and although it is often used with palliative intent, it may be used in an attempt to achieve a resectable state (1). Adam et al. (1) reported that resectability is successfully attained in only 12.5% of patients with initially unresectable colorectal hepatic metastases using modern combination chemotherapy.
The in vitro chemosensitivity test (CST) has been used in an attempt to select patient-specific chemotherapeutic regimens. The response rates seem to be better with in vitro-selected chemotherapeutic regimens than with empirical regimens, but the impact on survival has not been adequately addressed (2). The adenosine triphosphate-based chemotherapy response assay (ATP-CRA) was verified as a well documented and validated tool for individualizing chemotherapy and a survival benefit with this assay-guided chemotherapy design was observed in patients with unresectable small-cell lung cancer (3). There are several reports on complete remission of primary rectal cancer after preoperative chemoradiation (5, 6); however only a few case reports on colon cancer have been published (7).
We describe the first case of a patient, presented with sigmoid colon cancer with multiple liver and peritoneal metastases, in which there was a complete response for the primary colon cancer after administration of preoperative chemotherapy selected by ATP-CRA.
A 47-yr-old male was admitted to the hospital for hematochezia associated with a changing pattern of stools for 3 months in August 2005. Colonoscopy revealed an ulcerative, nearly-obstructing tumor in the sigmoid colon and biopsy confirmed a moderately differentiated adenocarcinoma. Abdominopelvic computed tomography (CT) (Fig. 1A, 2A) showed an exophytic tumor mass in the sigmoid colon, with invasion to the distal left ureter, multiple hepatic metastases (S8, S7, and S3), and multiple peritoneal seedings. The level of the serum carcinoembryonic antigen (CEA) was exceptionally high (12,292 ng/mL).
After deployment of a Hanaro Colorectal Stent® (M.I.Tech, Seoul, Korea) and insertion of a double J ureteral stent into the left ureter for the purpose of palliation, the patient was submitted for ATP-CRA with an informed consent (8). ATP-CRA using the patient's own tumor tissue demonstrated that oxaliplatin followed by 5-fluorouracil had the highest cell death rate (CDR), which represents the highest responsiveness (3) (Fig. 3). Twelve cycles of oxaliplatin-capecitabine chemotherapy, as a more convenient regimen for patient, were scheduled at three-week intervals (4). The patient was administered intravenous 230 mg (130 mg/m2/day) of oxaliplatin on day 1 every 3 weeks and 1,750 mg (1,000 mg/m2) of oral capecitabine twice a day on days 1-14 every 3 weeks. Treatment courses were well tolerated.
Abdominopelvic CT (Fig. 1B, 2B) performed after cycles 3, 6, and 9 revealed the shrinkage of the liver metastases (S8, 10.5 to 3.5 cm; S7, 7.2 to 3.4 cm; S3, 1.7 to 1.5 cm) but no definite interval change in the sigmoid colon cancer. Positron emission tomography (PET) (Fig. 4) showed a localized uptake of 18F-fluoro-deoxy-glucose (FDG) in the sigmoid colon and no uptake in the liver or peritoneum. The serum CEA was decreased to 5.1 ng/mL. Because there was a possibility for curative operation of the sigmoid colon and liver, the patient underwent an anterior resection, radiofrequency ablation of the hepatic metastases, and left nephrectomy for hydronephroureter in September 2006, which achieved a macroscopic R0 resection. No metastasis to the peritoneum was detected during the operation. Histological examination revealed no residual cancer cells in the resected specimen (CR) (Fig. 5). His last serum CEA level checked in March 2007 was found to be normal (2.2 ng/mL) and the patient was doing well with no evidence of disease 10 months after surgery and 25 months after the diagnosis of unresectable sigmoid colon cancer.
The use of systemic chemotherapy in patients with unresectable metastatic colorectal cancer has been traditionally regarded as a palliative approach. However, it has been thought of as a neoadjuvant approach to render patients resectable because of encouraging results of randomized trials using newly-developed chemotherapeutic agents. For more than three decades, 5-fluorouracil (5-FU) was the chemotherapeutic agent of choice with a response rate of approximately 20% (9). Recent chemotherapy combining 5-FU/leucovorin with either irinotecan or oxaliplatin has been demonstrated superior response rates (31-56%) compared with 5-FU alone (5%) (10, 11). Tournigand et al. (11) reported response rates of 54 and 56% in patients who received oxaliplatin with 5-FU (FOLFOX6) and irinotecan with 5-FU (FOLFIRI), respectively. However, they also demonstrated that these high response rates seen with first-line chemotherapy fell dramatically after second-line chemotherapy (15% FOLFOX6 and 4% FOLFIRI) and the survival for patients who underwent the second-line chemotherapy was also quite poor. Although these studies have not focused directly on the primary tumor, the higher rates of response and survival of the different combinations for metastatic lesions suggests that a direct effect on the primary lesion cannot be excluded. Therefore, chemotherapeutic agents should be carefully chosen because of their critical effect on the prognosis of the patient. This is the first to report a complete remission of primary colon cancer after preoperative combination chemotherapy selected by ATP-CRA.
The ATP-CRA is a sensitive assay that evaluates tumor cell viability by measuring the intracellular ATP levels of drug-exposed cells and untreated controls (3, 8). Moon et al. (3) showed good correlations between ATP-CRA results and clinical outcomes in patients with unresectable lung cancer. This case report highlights a new and different perspective in the management of patients with unresectable metastatic colorectal cancer. It is suggested that preoperative chemotherapy regimen chosen by ATP-CRA may be useful for treating these patients, although the results of in vitro CST only validated the use of the same set of drugs that would have been selected on the basis of the clinical trial literature (12). Further good correlations between ATP-CRA results and clinical outcomes are warranted.
Figures and Tables
Fig. 1
Multiple liver metastases in sigmoid colon cancer. (A) Before chemotherapy. (B) After chemotherapy.
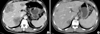
Fig. 2
Primary sigmoid colon cancer. (A) Before chemotherapy, an exophytic tumor mass (arrow) invading the distal left ureter. (B) After chemotherapy, a slight decrease in the size of the tumor mass (arrow) but no definite interval change. A Hanaro Colorectal Stent® and a double J ureteral stent were visible.

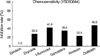
Fig. 3
Tumor suppression rate at peak plasma concentration (PPC). Longer bars indicate greater in vitro tumor suppression (p=NS).
ACKNOWLEDGEMENT
The authors would like to thank Sung Ho Choi, Ph.D., for providing technical assistance and advice.
References
1. Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghemard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004. 240:644–657.
2. Cortazar P, Johnson BE. Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol. 1999. 17:1625–1631.

3. Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, Park MS, Chung KY, Lee HJ, Kim JH. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007. 109:1829–1835.

4. Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, Schoffski P, Sobrero A, Van Cutsem E, Diaz-Rubio E. XELOX (capecitabine plus oxalipatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004. 22:2084–2091.
5. Guillem JG, Puig-La Calle J Jr, Akhurst T, Tickoo S, Ruo L, Minsky BD, Gollub MJ, Kilmstra DS, Mazumdar M, Paty PB, Macapinlac H, Yeung H, Saltz L, Finn RD, Erdi Y, Humm J, Cohen AM, Larson S. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000. 43:18–24.

6. Kaneki T, Koizumi T, Kawashima A, Tsushima K, Kubo K, Fujimoto K, Honda T, Akamatsu T. Double cancer (lung and colon cancer) that showed complete remission with irinotecan and cisplatin combined chemotherapy. J Gastroenterol. 2000. 35:864–869.

7. Brandi G, Pantaleo MA, Calabrese C, Di Battista M, Poggi R, Bajetta E, Biasco G. Complete remission of primary colon cancer in a metastatic patient treated with CPT-11 plus capecitabine. Int J Colorectal Dis. 2004. 19:599–602.

8. Huh JW, Park YA, Sohn SK, Choi SH. In-vitro chemosensitivity test for colorectal cancer using an adenosine-triphosphate-based chemotherapy response assay (ATP-CRA). J Korean Soc Coloproctol. 2007. 23:172–179.

9. Meta-analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998. 16:301–308.
10. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004. 22:23–30.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download