Abstract
The diagnostic accuracy of percutaneous transhepatic cholangioscopy (PTCS) was compared to that of three radiologic modalities in distal common bile duct (CBD) strictures for the evaluation of clinical application. Ninety-five patients who underwent PTCS for the evaluation of distal CBD strictures (35 malignant and 60 benign) whose masses were not obvious from radiologic imagings were included. Confirmative diagnosis could not be reached by endoscopic retrograde cholangiopancreatography (ERCP) or radiologic findings in all cases. Specific findings on the computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP) and direct cholangiography were analyzed among 68 (25 malignant and 43 benign) out of the 95 patients in order to determine the sensitivity and specificity of three radiologic studies for the diagnosis of malignant distal CBD strictures, and to compare those results with those by a combination of PTCS-guided biopsy and tumor vessel observation on cholangioscopy. The sensitivity/specificity of CT, MRCP and direct cholangiography including ERCP in diagnosing malignant distal CBD strictures were 42.9%/65.8%, 53.3%/58.3%, and 70.8%/47.6% respectively, while it was 96%/100% for the combination of PTCS-guided biopsy and tumor vessel. PTCS is a useful method for differential diagnosis of distal CBD strictures, particularly when it is difficult to distinguish benign from malignant strictures by radiologic studies and when peroral approach is not feasible.
Various inflammatory diseases and benign or malignant tumors are known to cause distal common bile duct (CBD) strictures (1-3). It is important to determine whether a biliary stricture has a benign or malignant cause as each requires a different treatment approach. Furthermore, as a normal distal CBD may show a narrow distal segment, it is difficult to assess the clinical significance of a distal CBD narrowing.
Generally, endoscopic retrograde cholangiopancreatography (ERCP) has been considered the method of choice for diagnosing biliary strictures due to its accuracy in establishing the site and cholangiographic features of strictures (4). However, there are some conditions that ERCP could not be successfully completed such as previous stomach surgery. In addition, other diagnostic approaches are required to evaluate the biliary stricture when tissue sampling is difficult using ERCP.
Percutaneous transhepatic cholangioscopy (PTCS) might be useful for the diagnosis and treatment of biliary strictures (5, 6). However, there are no published studies examining the usefulness of PTCS for evaluating distal CBD strictures.
The usefulness of PTCS for differential diagnosis of distal CBD strictures was evaluated in the present study. In addition, PTCS, computed tomography (CT), magnetic resonance cholangio pancreatography (MRCP) and direct cholangiography including ERCP were compared in terms of their accuracy in diagnosing distal CBD strictures.
Between January 1995 and December 2004, 95 consecutive patients who underwent PTCS for the evaluation of distal CBD strictures at Asan Medical Center, Seoul, Korea, were enrolled in this study. The medical records and radiologic findings were reviewed and clinical information was collected retrospectively. Distal CBD stricture was diagnosed by endoscopic retrograde cholangiography or cholangiograms obtained by means of percutaneous transhepatic biliary drainage (PTBD) tubes, and radiologic finding including CT or MRCP.
If the malignant distal CBD stricture was obvious, these patients promptly sent for operation. However, patients whose other masses were unable to be seen through radiologic findings and the cases of which the nature of distal CBD stricture was inconclusive as to benign or malignant underwent PTCS.
Cases in which malignancy could not be proven by tissue biopsy results were excluded from the malignant group, even if their strictures were clinically suspicious for malignancy. Those patients with distal CBD stricture, which was diagnosed by postoperative cholangioscopy via T-tube tract, were also excluded.
Malignant distal CBD strictures were confirmed when malignant cells were observed in specimens obtained using PTCS-guided biopsy or surgery. The malignant distal CBD stricture group comprised of 35 patients (22 men and 13 women; mean age, 66 yr) and the diagnosis of malignancy was confirmed by surgical resection with or without PTCS-guided biopsy in 29 and only PTCS-guided biopsy in 6. Strictures were considered benign when malignant cells were not found in resected specimens from the patients who underwent surgery or when there was no clinical and radiology evidence of disease progression during more than 6 months of follow-up in patients with negative PTCS-biopsy findings who did not undergo surgery. The benign distal CBD stricture group comprised of 60 patients (45 men and 15 women; mean age, 61 yr) and the median follow-up period for the benign group was 26 months (up to 60 months).
In our study, 59 out of 95 patients could not undergo successful ERCP due to a previous history of Roux-en-Y esophagojejunostomy or gastrojejunostomy (n=31), cannulation failure (n=19), cardiovascular disease (n=4), difficulty to assume a lateral position (n=2) and others (n=3). Six patients underwent PTCS instead of ERCP, because ERCP had been performed at another hospital for evaluation of indeterminate distal CBD stricture suspicious for malignancy and they were referred to our tertiary referral center for cholangioscopy. Thirty patients underwent ERCP and in 8 out of 30 patients, we could not get the ERCP-guided biopsy and brush cytology due to tight distal CBD structures (n=5), hemobilia (n=1) and others (n=2). In 8 patients, ERCP-guided tissue sampling of distal CBD stricture was not done and we could not find the cause. The other 14 patients were unsuccessfully diagnosed because no additional information was obtained even though an ERCP-guided biopsy and a brush cytology were done. Consequently confirmative diagnosis could not be reached by ERCP alone in all cases.
PTCS was performed as inpatients procedures. PTBD was performed using a 8.5-Fr pigtail catheter under fluoroscopic guide. Two or three days after the PTBD, the percutaneous tract was dilated for the passage of the cholangioscope usually by one-stage dilation (eg, from 8.5 F to 16 or 18 F). For the sinus tract maturation, 7-10 days were required for the diagnostic cholangioscopy. PTCS was performed using a 5.2 mm outer diameter cholangioscope (CHF P20Q: Olympus Optical Ltd, Tokyo, Japan) or 4.9 mm outer diameter cholangioscope (FCN-15X; Pentax, Tokyo, Japan) with medication of meperidine (25-50 mg) and/or midazolam (3-5 mg). PTCS was performed by 3 endoscopists who had PTCS experience of at least 40 cases yearly for 2-7 yr. The distal CBD strictures were carefully examined for the presence of abnormal dilated vessel and mucosal abnormality. Targeted biopsies were performed with a 1.8 mm diameter forceps (FB-19SX01, Olympus) under direct vision.
Laboratory data such as CA 19-9 and carcinoembryonic antigen (CEA) concentrations were compared, and CT, MRCP, direct cholangiography findings and PTCS findings were analyzed. Direct cholangiography was defined as endoscopic retrograde cholangiography or cholangiograms obtained by means of PTBD tubes. Percutaneous transhepatic cholangiogram data was analyzed when ERCP was unsuccessful.
Mucosal findings such as tumor vessel, mucosal irregularity and mucosal color change were compared on cholangioscopy. A tumor vessel was defined as an abnormally proliferating and tortuous vascular structure in the bile duct stricture mucosa. Mucosal irregularity was defined as granular mucosa, nodular mucosa, papillary mucosa, mucosal hyperplasia and irregular mucosa. Mucosal color change was defined as whitish or hyperemic mucosa. PTCS-guided biopsies were performed at the sites of stricture, tumor vessel, mucosal irregularity and mucosal color change and three more biopsy specimens per patient were obtained. The sensitivity and specificity of PTCS-guided biopsy were calculated for the diagnosis of malignant versus benign distal CBD stricture. Additionally the sensitivity and specificity of combination of PTCS-guided biopsy and tumor vessel on cholangioscopy were compared with those by CT, MRCP and direct cholangiography for diagnosis of malignant distal CBD stricture.
The radiologist reviewed all radiologic images retrospectively by using a picture archiving and communication system (PACS) workstation monitor. The reviewer had no knowledge of clinical data or whether distal CBD strictures were diagnosed as malignant or benign following surgery or biopsy. The proportion of patients who underwent all three types of imaging study (CT, MRCP and direct cholangiography) was relatively small, thus the analysis included patients that underwent two or more imaging studies, which comprised 68 of the 95 patients (25 malignant, 43 benign). Twenty-seven patients were excluded from this analysis either because there were no images before decompression, the images were older than the obligatory storage period expiry date (5 yr) or the quality of outside images was very poor.
A stricture was assumed to be malignant if characterized by wall thickening, enhancement and abrupt narrowing according to the CT, and an irregular margin, asymmetric and abrupt narrowing according to MRCP and direct cholangiography. The sensitivity and specificity of diagnosis using CT, MRCP and direct cholangiography for malignant distal CBD strictures were determined.
All procedures were explained to patients, and all patients gave informed written consent to the procedures. The study was approved by the Institutional Review Board of Asan Medical Center.
All statistical analyses were performed using the SPSS 12.0 statistical software program (SPSS, Inc., Chicago, IL, U.S.A.). Potential predictive factors for PTCS findings that could be diagnosed as a malignant distal CBD stricture were analyzed with chi-square tests for univariate comparisons. Factors that were deemed of potential importance on univariate analysis (p<0.05) were included in the multivariate analysis. Logistic regression was used for multivariate analysis of these factors. Student's t-test was used to test for the differences between the benign and malignant groups in terms of laboratory test results, stricture length and upstream ductal diameters according to the CT. Chi-square tests were used to compare the radiograpic findings according to MRCP and direct cholangiography. A p value of <0.05 was considered to indicate a significant difference.
Baseline clinical findings are summarized in Table 1. The mean serum values for alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT) and total bilirubin were higher in the malignant than those in the benign group (p< 0.05). The mean CA 19-9 levels of the benign and malignant groups were 227 μ/mL and 396 μ/mL, respectively, without a significant difference between the two groups (Table 2).
The PTCS findings for the malignant and benign groups were compared. PTCS showed tumor vessels in 26, mucosal irregularity in 21 and mucosal color change in 12 of 35 malignant distal CBD stricture patients, while tumor vessels were not observed in benign groups and mucosal irregularity and mucosal color change were seen in 21 and 8 of 60 benign stricture patients. On univariate analysis, malignant distal CBD stricture was associated with tumor vessel, mucosal irregularity and mucosal color change (p<0.05). However, multivariate stepwise logistic regression analysis showed that the tumor vessel was only an independently differentiating variable (p<0.05) (Table 3).
In 35 patients of the malignant group, 33 underwent a PTCS-guided biopsy. For the remaining 2 patients, while biopsies were attempted up to four times, adequate biopsy specimens could not be obtained, and the malignancy was confirmed following surgical resection. Of the 3 patients of malignant group, PTCS-guided biopsy diagnosed their strictures as chronic inflammation. Therefore, five patients could not be diagnosed by PTCS-guided biopsy. Sixty patients of benign group underwent PTCS-guided biopsy and they were diagnosed with benign distal CBD stricture. Those patients with benign distal CBD strictures showed no significant interval change during the at least 6 months follow-up period.
The diagnostic accuracies associated with PTCS-guided biopsy and tumor vessel are shown in Table 4. The most valuable cholangioscopic finding for indicating the presence of a malignant distal CBD stricture was found to be the tumor vessel. Furthemore, a combination of tumor vessel findings and PTCS-guided biopsy resulted in a superior preoperative diagnosis compared to tumor vessel or PTCS-guided biopsy alone, which provided 97.1% sensitivity, 100% specificity, 100% positive predictive value and 98.4% negative predictive value (Table 4).
The sensitivity and specificity of combined PTCS-guided biopsy plus tumor vessel were compared with those of CT, MRCP and direct cholangiography for the diagnosis of malignant distal CBD strictures (Table 5). Sixty-eight patients were included in this analysis. The sensitivity/specificity of CT, MRCP and direct cholangiography were 42.9%/65.8%, 53.3%/58.3% and 70.8%/47.6%, respectively, while it was 96%/100% for the combined assessment using tumor vessel and PTCS-guided biopsy findings. Among 68 patients, 23 patients were suspected of malignancy according to at least one of CT, MRCP or direct cholangiographic images, and also shown to have malignant distal CBD strictures according to PTCS findings and biopsies. Sixteen patients showed benign radiologic features in distal CBD strictures and also showed benign PTCS findings and biopsy results. For 27 patients, while radiologic images indicated malignant distal CBD strictures, PTCS and biopsy findings revealed benign strictures (Fig. 1). For those 27 patients, the mean values for ALP, GGT and total bilirubin were 251 IU/L±221.6, 205 IU/L±198.5 and 3.2 mg/dL±4.96, respectively, and these mean values were significantly lower than those of the malignant distal CBD stricture group (p<0.05). For one patient, radiology showed a benign stricture while PTCS and biopsy findings showed a malignant stricture (Fig. 2).
Three of 95 patients experienced some complications including deep sedation (n=1) and minor bleeding (n=2) during cholangioscopy. They recovered following conservative management and there was no other complication such as pancreatitis or cholangitis.
The present retrospective study showed that combined use of PTCS-guided biopsy and tumor vessel observation resulted in a highly accurate method for diagnosing distal CBD strictures. This approach was superior to the use of CT, MRCP and direct cholangiography. These observations show that PTCS would be useful in diagnosing distal CBD strictures in cases that radiologic findings are ambiguous or ERCP techniques are inadequate.
After the mid 1970's, PTCS was predominantly performed in Asian countries such as Taiwan, Korea and Japan, where there had been a high prevalence of intrahepatic stones and cholangiocarcinomas (7, 8). The disadvantages of this procedure are that it is invasive and requires sinus tract dilatation which takes a relatively long time (9). However, the advantages are direct visualization of the biliary mucosa and the ability to perform a PTCS-guided biopsy (10, 11).
The sensitivity of PTCS-guided biopsy was reported as 80-89% (12, 13), and the present study found it to be 85.7%. The previous prospective studies reported that the sensitivity of ERCP-guided biopsy and brush cytology was 61-75% for biliary tumors (14, 15). In some cases of distal CBD stricture, ERCP-guided biopsy is more technically difficult. Thus, cholangioscopic biopsy appears to be very useful since a clinician can precisely perform a targeted mucosal biopsy during continuous observation. In the current study, the number of patients who underwent ERCP-guided biopsy and brush cytology was so small that we could not compare ERCP with PTCS in terms of tissue diagnosis.
Sato et al. has also reported that PTCS-guided biopsy can detect invasive carcinomas only in the superficial layers of the bile duct and diagnosis of intramural extension of invasive carcinoma is difficult with PTCS even when the lesion is biopsied (16). Therefore, reliable diagnostic criteria are required for accurate differential diagnosis in cases of difficult biopsies. PTCS is a useful method for distinguishing cholangiocarcinomas from other benign CBD strictures as it provides direct visualization of any tumor vessels. In the bile duct mucosa, the tumor vessel is an abnormal tortuous and serpentine vascular structure at the circumference of the cancer mucosa (17), and is important for tumor nutrition. Such a vessel does not exist on a benign mucosal surface.
Of the 35 malignant group patients, biopsy results showed chronic inflammation in 3 patients, and adequate biopsy specimens could not be obtained in 2 patients. Five patients could not initially be confidently placed in either the malignant or benign group and the malignant distal CBD strictures all were confirmed by surgical resection. Four of those 5 patients had tumor vessels and only tumor vessel was associated with malignant distal CBD stricture on multivariate analysis. Therefore, it appears that the presence of a tumor vessel is a valuable clue for identifying malignancy. It is meaningful that observation of a tumor vessel in distal CBD strictures by PTCS because observation of a tumor vessel by ERCP is impossible.
The present study documented PTCS-guided biopsy findings combined with tumor vessel observation provided the most precise preoperative diagnosis. This observation is consistent with that of a previous study involving 41 malignant biliary stricture patients that reported that the sensitivity of tumor vessel observation was 61%, the sensitivity of PTCS-guided biopsy findings was 80%, while the sensitivity of the two methods combined were 96% (12).
In the present study, patients who failed ERCP due to unsuccessful cannulation did not undergo peroral cholangioscopy which would have been much less invasive. In addition, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for the evaluation of distal CBD strictures was not performed.
The diagnostic utility of peroral cholangioscopy has not yet been examined vigorously and the observation of far distal CBD on peroral cholangioscopy is techniqually difficult. The fragility of the equipment and technical difficulties hold back its popularity.
EUS-FNA has been shown, in many series, to be highly accurate for the diagnosis of pancreatic masses; sensitivity rates for malignancy range from 60% to 93% (18-23). In a prospective study, it has been demonstrated that the sensitivity of EUS-based techniques for the diagnosis of malignant biliary stricture was only 25% and EUS-guided biopsy was superior for pancreatic mass (14). In that study, if a mass lesion was noted around the bile duct, EUS-FNA was attempted and if a circumscribed mass was not demonstrated by EUS, needle puncture was not attempted. There is few published data in respect to EUS-guided tissue sampling of distal CBD stricture without definite CBD mass on radiologic imaging. EUS-FNA, therefore, may not be more valuable than PTCS with biopsy for the evaluation of distal CBD stricture without visible tumor in distal CBD.
Guidelines need to be developed to determine which distal CBD stricture cases require PTCS. We suggest PTCS be undertaken in patients in whom it is difficult to determine whether the distal CBD strictures are benign or malignant by radiological findings and ERCP and get ERCP-guided tissue sampling are not feasible: Billroth type II surgery, cannulation failure, tight distal CBD strictures and etc.
The present study was retrospective and therefore has the limitations inherent in such a study design. In addition, the number of patients who underwent all three imaging procedures (CT, MRCP and direct cholangiography) was relatively small, so analysis involved patients who had undergone at least two of the three procedures. Furthermore, on the interpretation of CT, MRCP and direct cholangiography findings for distal CBD strictures, it is likely that observational disagreement for the evaluation of the results exist. Therefore, the evaluation of these imaging modalities by one radiologist may be biased. Moreover, sensitivity of CT may be underestimated because we did not adjust for the fact that, prior to March 2001, evaluation of biliary tract disease was performed by conventional abdomen CT, which was later replaced by dynamic biliary CT. Thus, the outcomes of this study must be considered in the context of these limitations.
In conclusion, the present study found that due to its ability to provide direct visualization of the mucosa and any tumor vessels, and to its ability to guide biopsies, PTCS was a useful method for identifying malignant distal CBD strictures compared to CT, MRCP and direct cholangiography. In particular, we recommend PTCS to obtain an accurate diagnoses in cases where laboratory findings, radiographic findings and tissue sampling results following ERCP are inconclusive to exclude a malignant distal CBD stricture.
Figures and Tables
Fig. 1
Studies of a 59-yr-old male patient. While radiologic findings suggested a malignant distal common bile duct stricture, PTCS and biopsy findings revealed a benign stricture. (A) MRCP image showing abrupt and asymmetric narrowings. (B) ERCP image showing abrupt and asymmetric narrowings. (C) PTCS image showing no tumor vessel or mucosal abnormality.
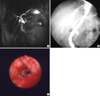
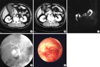
Fig. 2
Studies of a 64-yr-old male patient. While radiologic findings suggested a benign stricture, PTCS imaging showed hyperemic mucosa and tumor vessels, and PTCS-guided biopsy data revealed an adenocarcinoma. (A, B) CT image showing no wall thickening or enhancement. (C) MRCP image showing gradual narrowing and symmetric narrowing. (D) Percutaneous transhepatic cholangiogram image showing gradual narrowing and smooth narrowing. (E) PTCS image showing hyperemic mucosa and tumor vessels in the distal common bile duct.
References
1. Nimura Y. Staging of biliary carcinoma: cholangiography and cholangioscopy. Endoscopy. 1993. 23:76–80.

2. Van Steenbergen W, Van Aken L, Van Beckevoort D, Stockx L, Fevery J. Percutaneous transhepatic cholangioscopy for diagnosis and therapy of biliary diease in older patients. J Am Geriatr Soc. 1996. 44:1384–1387.
3. Yeh YH, Huang MH, Yang JC, Mo LR, Lin J, Yueh SK. Percutaneous transhepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995. 42:13–18.

4. Hawes RH. Diagnostic and therapeutic uses of ERCP in pancreatic and biliary tract malignancies. Gastrointest Endosc. 2002. 56:S201–S205.

5. Nimura Y, Kamiya J, Hayakawa N, Shionoya S. Cholangioscopic differentiation of biliary strictures and polyps. Endoscopy. 1989. 21:Suppl 1. 351–356.

7. Nakajima M, Akasaka Y, Fukumoto K, Mitsuyoshi Y, Kawai K. Peroral cholangiopancreatoscopy (PCPS) under duodenoscopic guidance. Am J Gastroenterol. 1976. 66:241–247.
8. Urakami Y, Seifert E, Butke H. Peroral direct cholangioscopy (PDCS) using routine straight-view endoscope: first report. Endoscopy. 1977. 9:27–30.

9. Frimberger E, Vente T, Wagenpfeil S, Gerein P, Born P, Fritz N, Allescher HD, Ott R, Weigert N, Classen M, Rosch T. A new system for rapid large-caliber percutaneous transhepatic drainage in patients with obstructive jaundice: a prospective randomized trial. Endoscopy. 2001. 33:201–209.

10. Kim MH. Percutaneous transhepatic cholangioscopic examination: a necessity for the biliary endoscopist. Gastrointest Endosc. 2001. 53:695–697.

11. Classen M, Neuhaus H. Diagnostic and therapeutic peroral and percutaneous cholangioscopy. J Gastroenterol. 1994. 29:Suppl 7. 143–147.
12. Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000. 52:635–638.

13. Hwang MH, Tsai CC, Chou CY, Mo LR, Yang CT, Lin RC, Yueh SK. Percutaneous cholangiofiberscopic endoluminal forceps biopsy of intrabile duct diseases. Hepatogastroenterology. 1998. 45:2073–2078.
14. Rosch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U, Werner M. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004. 60:390–396.
15. Pugliese V, Conio M, Nicolo G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary stricture: a prospective study. Gastrointest Endosc. 1995. 42:520–526.
16. Sato M, Inoue S, Ogawa , Ohashi S, Maetani I, Igarashi Y, Sakai Y. Limitations of percutaneous transhepatic cholangioscopy for diagnosis of the intramural extension of bile duct carcinoma. Endoscopy. 1998. 30:281–288.
17. Yamase H, Nimura Y, Hayakawa N. Differential diagnosis on stenosis of ductal bile duct by percutaneous transhepatic cholangioscopy (in Japanese). Gastroenterol Endosc. 1988. 30:175–182.
18. Bhutani MS, Hawes RH, Baron PL, Sanders-Cliette A, Van Velse A, Osborne JF, Osborne JF, Hoffman BJ. Endoscopic ultrasound (EUS) guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997. 29:854–858.
19. Binmoeller KF, Thul R, Rathod V, Henke P, Brand B, Jabusch HC, Soehendra N. Endoscopic ultrasound-guided, 18-gauge, fine-needle aspiration biopsy of the pancreas using a 2.8 mm channel convex echoendoscope. Gastrointest Endosc. 1998. 47:121–127.
20. Chang KJ, Katz KD, Durbin TE, Erickson RA, Butler JA, Lin F, Wuerker RB. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994. 40:694–699.

21. Fritscher-Ravens A, Sriram P, Krause C, Atay Z, Jaeckle S, Thonke F, Brand B, Bohnacker S, Soehendra N. Detection of pancreatic metastases by EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001. 53:65–70.

22. Giovannini M, Seitz JF, Monges G, Rabbia I, Perrier H. Fine-needle aspiration biopsy guided by endoscopic ultrasonography. Results in 141 patients. Endoscopy. 1995. 27:171–177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download