Abstract
Figures and Tables
Table 1
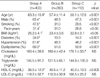
*p<0.001 vs. group B and group C; †p<0.05 vs. group C; ‡p<0.05 vs. group B and group C.
Group A, ≥50% in luminal diameter stenosis in coronary angiography; Group B, 20-50% in luminal diameter stenosis in coronary angiography; Group C, <20% in luminal diameter stenosis in coronary angiography. BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 2
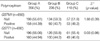
*Valid results of GSTM1/GSTT1 genotype were 692 of 775 subjects (89.3%).
Group A, ≥50% in luminal diameter stenosis in coronary angiography; Group B, 20-50% in luminal diameter stenosis in coronary angiography; Group C, <20% in luminal diameter stenosis in coronary angiography. GST, glutathione S-transferase.
Table 3

*reference.
Group A, ≥50% in luminal diameter stenosis in coronary angiography; Group B, 20-50% in luminal diameter stenosis in coronary angiography; Group C, <20% in luminal diameter stenosis in coronary angiography.
GST, glutathione S-transferase; CAD, coronary artery disease; OR, odds ratio; M1(+), GSTM1-positive genotype; M1(-), GSTM1 null genotype; NS, non-smoker; S, smoker.
Table 4

*, reference.
Group A, ≥50% in luminal diameter stenosis in coronary angiography; Group B, 20-50% in luminal diameter stenosis in coronary angiography; Group C, <20% in luminal diameter stenosis in coronary angiography.
GST, glutathione S-transferase; CAD, coronary artery disease; OR, odds ratio; T1(+), GSTT1-positive genotype; T1(-), GSTT1 null genotype; NS, non-smoker; S, smoker.
Table 5

*, reference; †, adjusted for age, sex, hypertension, DM, body mass index, and lipid profile.
Group A, ≥50% in luminal diameter stenosis in coronary angiography; Group B, 20-50% in luminal diameter stenosis in coronary angiography; Group C, <20% in luminal diameter stenosis in coronary angiography.
GST, glutathione S-transferase; CAD, coronary artery disease; OR, odds ratio; M1(+), GSTM1-positive genotype; M1(-), GSTM1 null genotype; NS, non-smoker; S, smoker.
Table 6

*reference; †, adjusted for age, sex, hypertension, DM, body mass index, and lipid profile.
Group A, ≥50% in luminal diameter stenosis in coronary angiography; Group B, 20-50% in luminal diameter stenosis in coronary angiography; Group C, <20% in luminal diameter stenosis in coronary angiography.
GST, glutathione S-transferase; CAD, coronary artery disease; OR, odds ratio; T1(+), GSTT1-positive genotype; T1(-), GSTT1 null genotype; NS, non-smoker; S, smoker.
Table 8

GST, glutathione S-transferase; CAD, coronary artery disease; mild, 50-75% of luminal diameter stenosis; moderate, 76-90% of luminal diameter stenosis; severe, >90% of luminal diameter stenosis; NS, non-smoker; S, smoker; GSTM1-1, GSTM1-positive genotype; GSTM1-0, GSTM1 null genotype; GSTT1-1, GSTT1-positive genotype; GSTT1-0, GSTT1 null genotype; both-1, GSTM1/T1 both positive genotype; both-0, GSTM1/T1 both null genotype.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download