Abstract
We analyzed the efficacy and toxicity of a modified Cancer and Leukemia Group B (CALGB) 19802 regimen in adult acute lymphoblastic leukemia (ALL). From February 2002 to August 2005, 25 adults with untreated ALL were enrolled in the study. Compared to the original regimen, the modified CALGB 19802 regimen consisted of a 4-drug induction (cyclophosphamide, daunorubicin, vincristine, and prednisone) instead of a 5-drug induction (L-asparaginase was added to the previous regimen). This was followed by high-dose methotrexate (1,000 mg/m2×3 days) and cytarabine (2,000 mg/m2×4 days) for the consolidation cycles. High-dose systemic and intrathecal methotrexate was given for central nervous system prophylaxis. Twenty-three patients (92%) achieved a complete remission (CR), and two patients (8%) had refractory disease. With a median follow-up of 21.5 months, 10 patients (40%) were alive and continued to be in CR. The 3-yr probability of an event-free survival and the overall survival were 39.0% and 47.4%, respectively. Treatment related mortality and major grade 3 to 4 neurotoxicity occurred in 1 patient and 3 patients, respectively. The modified CALGB 19802 regimen demonstrated a high remission rate and a favorable survival rate.
Although aggressive combination chemotherapy for adult acute lymphoblastic leukemia (ALL) has improved the complete response (CR) rates, long-term survival rates seldom exceed 40 percent (1-5). An intensive regimen developed by the Cancer and Leukemia Group B (CALGB) has shown an 85% CR rate. However, the overall survival (OS) rate has not improved significantly (1, 6). The CALGB study 19802 was started based on the hypothesis that a post-remission intensification regimen for eradicating the minimal residual disease could increase OS (7). The preliminary experience with the CALGB 19802 regimen for adults ALL demonstrated that the post-remission regimen was feasible with acceptable side effects (7).
The intensive use of L-asparaginase, during the postinduction period, was found to improve the cure rate in childhood ALL (8, 9). However, this agent is difficult to deliver to adults and also need frequent blood tests focusing on the coagulation system and serum amylase because of significant toxicity (5, 10-12). We evaluated a modified CALGB 19802 regimen that omitted L-asparaginase from the original CALGB 19802 regimen in adult ALL. Here, we present our results on the efficacy and toxicity of the modified CALGB 19802 regimen.
From February 2002 to August 2005, 25 adults with untreated ALL were enrolled in this study. The diagnosis of ALL was based on morphology, histochemical stains, and immunophenotyping. Cytogenetic and molecular analyses were performed. The clinical records were reviewed to evaluate treatment outcomes and toxicity.
Treatment consisted of six courses given in the order A-B-C-A-B-C followed by 18 months of a maintenance phase (7). Induction chemotherapy (course A) consisted of cyclophosphamide, daunorubicin, vincristine, and prednisolone. Granulocyte colony-stimulating factor (G-CSF) was started when the absolute neutrophil count (ANC) decreased below 500/µL. This was a modified CALGB 19802 regimen with the removal of L-asparaginase and change of the schedule for G-CSF administration.
Post-remission therapy included five courses of consolidation chemotherapy. Course B consisted of cyclophosphamide (1,000 mg/m2 on day 1) and high-dose cytarabine (2,000 mg/m2 on days 1-4). G-CSF was started when ANC decreased below 500/µL. Course C consisted of intravenous high-dose methotrexate (1,000 mg/m2 on days 1, 8, and 15) and vincristine (2 mg on day 1, 8, and 15). Oral methotrexate (25 mg/m2) was taken every six hours for four doses, starting 6 hr after the beginning of the intravenous methotrexate. Oral leucovorin (10 mg) was continued every 12 hr until the methotrexate levels measured fell below 0.05 µM.
At the end of 6 courses of A, B, and C chemotherapy, patients received maintenance chemotherapy on a monthly basis: prednisone 60 mg/m2 on day 1-5, intravenous vincristine 2 mg on day 1, oral methotrexate 20 mg/m2 weekly, and oral 6-mercaptopurine 60 mg/m2 daily.
CNS prophylaxis was provided with intrathecal methotrexate plus hydrocortisone. The first dose was given on day 1 of cycle B. The subsequent doses were given on days 1, 8, and 15 of cycle C with systemic high-dose methotrexate.
Bone marrow aspiration and biopsy were performed at the end of the induction phase. Patients were considered to be in CR when the ANC was more than 1,000/µL, the platelet count was higher than 100,000/µL, the bone marrow morphology was normal with less than 5% blasts, there was no evidence of extramedullary leukemia, and there was resolution of the previously abnormal cytogenetics.
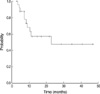
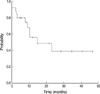
Event-free survival (EFS) was defined as the time from diagnosis to induction failure, relapse, or death. Patients who did not achieve remission were included in the analysis and considered as treatment failures with an EFS of 0 days. OS was measured from the date of diagnosis to the date of death or the last follow-up visit. Six patients received stem cell transplantation (SCT) were censored. The Kaplan-Meier product-limit method was used to estimate the EFS and the OS. The survival rates were compared using the log-rank test. Differences were considered statistically significant when p<0.05.
Clinical and hematological characteristics of the 25 patients are summarized in Table 1. The median age of all patients was 45 yr (range, 18 to 68 yr), and five (20%) patients were older than 60. Hepatosplenomegaly was present in 10 (40%) patients. Five (20%) patients had mediastinal involvement. Two patients (8%) had evidence of CNS leukemia at the time of diagnosis. The median initial WBC count was 12.24×109/L (range, 2.0-149), and eight (32%) patients had an initial WBC count higer than 30×109/L. Immunophenotype analysis showed that 18 (72%) patients had B-lineage ALL and 7 (28%) patients had T-lineage ALL. Chromosomal analysis was available in all patients, and the philadelphia (Ph) chromosome was found in nine (36%) patients. No patient had t(4;11).
CR was achieved in 23 patients (92%), while 2 (8%) had refractory disease. Remission was achieved with the first course of treatment in 22 patients and required a second course of treatment in one. The median time to achieve CR was 24 days (range, 17-86).
Among the 23 eligible patients who achieved CR, three patients could not receive the consolidation therapy due to relapse (n=2) or infection (n=1). Among the 20 patients who received the consolidation therapy, only 10 patients completed the consolidation therapy. The reasons for an incomplete consolidation therapy were stem cell transplantation in 6 patients, death in 1 patient, drug toxicity in 1 patient, and relapse during consolidation in 2 patients. Among the 10 patients who completed the consolidation therapy, six patients did not enter the maintenance therapy due to relapse (n=3), drug toxicity (n=2), or patient refusal (n=1). Of the four patients who entered the maintenance therapy, three patients completed maintenance therapy. One patient could not complete the maintenance therapy due to death.
At a median follow-up period of 22 months (range, 2 to 47 months), 10 patients (40%) are alive and continue to be in CR. Three patients (12%) died in remission. Seven patients (30%) among 23 patients who achieved CR relapsed. There was no case of an isolated CNS relapse. Six patients (24%) received SCT during the first CR: 5 allogenic SCT, 1 autologous SCT. One patient (4%) received SCT after relapse. Patients who received SCT were censored at the time of the transplantation. Of the nine patients with a Ph chromosome, three patients were treated with imatinib mesylate. Among them, two patients received imatinib mesylate monotherapy after reinduction chemotherapy due to relapse (n=1) and refractory state (n=1), and 1 patient received imatinib mesylate monotherapy after induction. For all 25 patients, the estimated 3-yr EFS and OS were 39.0% and 47.4%, respectively (Fig. 1, 2).
There were no significant prognostic factors identified in the univariate and multivariate analysis (Table 2).
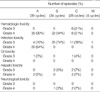
The major toxicity after induction and consolidation chemotherapy was hematotoxicity (Table 3). Hematotoxicity occurred frequently during cycles A and B. The median time to recovery of the ANC to higher than 1,000/µL was 16, 17, and 20.5 days during cycles A, B, and C, respectively. The median time to recovery of the platelet count to higher than 100,000/µL was 22.5, 26.5, and 33 days during cycles A, B, and C, respectively. The second most common toxicity was infection. There were 33 documented infections (19 bacterial, 8 fungal, and 6 viral infections) and 33 cases of febrile neutropenia (fever of unknown origin without documented infection). Both the frequency and severity of the infections were highest during cycle A. Neurological toxicity occurred in 3 patients. Among them, one patient developed cerebellar dysfunction attributable to the high-dose cytarabine during the first cycle B. In addition, two patients developed myelopathy after intrathecal methotrexate administration during the second cycle C. Although they received the appropriate management, none of them improved and they could not continue chemotherapy.
The CALGB 19802 regimen is characterized by early dose intensification of daunorubicin during induction and post-remission therapy and high-dose cytarabine and methotrexate during post-remission therapy. This regimen aims to improve survival in adults with ALL by dose intensification using agents already known to be effective in ALL (7). In a recent update of the CALGB 19802 regimen, the median survival period was 1.6 yr and the median disease-free survival period was 1.5 yr. This regimen showed no improvement in the OS and the EFS compared with other studies (13-17). Although our modified CALGB 19802 regimen omitted L-asparaginase from the CALGB 19802 regimen, it followed the characteristics of original regimen, identically. In our study, the modified CALGB 19802 regimen resulted in a high CR rate and a favorable EFS and OS. The difference in outcomes, compared with the original CALGB 19802 regimen, could be due to one or more of the following factors: a small number of patients, no removal of cyclophosphamide from the A cycle in older patients, or the inclusion of patients who had SCT. Recent nonrandomized studies showed that intensive consolidation therapy improved the outcome with the use of cyclophosphamide and high-dose cytarabine (10, 18). However, intensified anthracycline has shown no clear benefit in adults (3, 19). Considering the small number of patients and the retrospective nature of the study, the value of the individual components of the modified 19802 regimen could not be confirmed in this study.
L-asparaginase has an established role in the treatment of childhood ALL (9, 20). Although several clinical trials using L-asparaginase in adult ALL have been reported, it is difficult to evaluate the true impact of L-asparaginase in the treatment strategy for adult ALL because L-asparaginase was given with other drugs simultaneously (21, 22). Nagura et al. showed no significant difference in the CR rate and the OS between induction chemotherapy with and without L-asparaginase in a randomized clinical trial on adult ALL (23). These findings are consistent with our results; our study showed no significant difference in the CR rate and the OS compared with other studies using L-asparaginase (3, 6, 24). However, a recent study showed that effective asparagines depletion with pegylated asparaginase, as part of an intensive multiagent therapeutic regimen in adult ALL, was feasible and associated with improved outcomes (25). In comparison with previous studies, this discrepancy may be attributed to differences in dose intensity and duration of asparaginase therapy. Larger patient cohorts are required to determine the true impact of L-asparaginase in the treatment of adult ALL.
There was no CNS relapse in cases without cranial radiation for CNS prophylaxis. However, grade 4 neurotoxicity occurred in three (12%) patients. Among them, cerebellar toxicity due to cytarabine was found in a 66-yr-old man after receiving his first course of cycle B chemotherapy. Two other patients had myelopathy after receiving intrathecal methotrexate during the second cycle C. Although it was difficult to determine the precise incidence of the neurotoxicities for the ALL therapy in adults due to the heterogeneity, grade 3 to 4 neurotoxicities occurred more frequently and were more severe than in other studies (7, 10, 18, 26). Considering the high median age of the patient population, the CNS toxicity with high-dose cytarabine was acceptable. However, we cannot account for the high occurrence of the neurotoxicity related to methotrexate because the etiology of methotrexate toxicity remains uncertain (27). Further studies are necessary to evaluate the prognostic factors for neurological events linked to methotrexate.
The modified CALGB19802 regimen produced a high remission rate and a favorable survival rate. However, this study has several limitations including the small number of patients, the retrospective study design, and the relatively short follow-up duration. Therefore, further studies with a longer follow-up and randomized study design are needed to confirm the benefit on OS and EFS.
Figures and Tables
References
1. Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, Duggan D, Davey FR, Sobol RE, Frankel SR, Hooberman AL, Westbrook CA, Arthur DC, George SL, Bloomfield CD, Schiffer CA. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995. 85:2025–2037.

2. Kantarjian HM, Walters RS, Keating MJ, Smith TL, O'Brien S, Estey EH, Huh YO, Spinolo J, Dicke K, Barlogie B, McCredie KB, Freireich EJ. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol. 1990. 8:994–1004.

3. Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, Ferrara F, Peta A, Ciolli S, Deplano W, Fabbiano F, Sica S, Di Raimondo F, Cascavilla N, Tabilio A, Leoni P, Invernizzi R, Baccarani M, Rotoli B, Amadori S, Mandelli F. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002. 99:863–871.

4. Garcia-Manero G, Kantarjian HM. The hyper-CVAD regimen in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000. 14:1381–1396.

5. Hoelzer D, Gokbuget N. New approaches to acute lymphoblastic leukemia in adults: where do we go? Semin Oncol. 2000. 27:540–559.
6. Larson RA, Dodge RK, Linker CA, Stone RM, Powell BL, Lee EJ, Schulman P, Davey FR, Frankel SR, Bloomfield CD, George SL, Schiffer CA. A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood. 1998. 92:1556–1564.
7. Cataland SR, Daugherty CK, Weseman EC, Larson RA. Preliminary experience with a new chemotherapy regimen for adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2001. 41:297–307.

8. Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Arkin S, Declerck L, Cohen HJ, Sallan SE. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001. 97:1211–1218.
9. Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, van Wering ER, Lo Nigro L, van der Does A, Locatelli F, Basso G, Arico M. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005. 23:7161–7167.

10. Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeha S, Bueso-Ramos CE, Pierce S, Shan J, Koller C, Beran M, Keating M, Freireich EJ. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004. 101:2788–2801.

11. Chim CS, Kwong YL, Chu YC, Chan CH, Chan YT, Liang R. Improved treatment outcome in adult acute lymphoblastic leukemia using the intensive German protocol, a preliminary report. Hematol Oncol. 1997. 15:19–26.

12. Kieslich M, Porto L, Lanfermann H, Jacobi G, Schwabe D, Bohles H. Cerebrovascular complications of L-asparaginase in the therapy of acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2003. 25:484–487.

13. Durrant IJ, Richards SM, Prentice HG, Goldstone AH. The Medical Research Council trials in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000. 14:1327–1352.

14. Thiebaut A, Vernant JP, Degos L, Huguet FR, Reiffers J, Sebban C, Lepage E, Thomas X, Fiere D. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am. 2000. 14:1353–1366.
15. Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000. 14:1367–1379.

16. Hoelzer D, Thiel E, Loffler H, Buchner T, Ganser A, Heil G, Koch P, Freund M, Diedrich H, Ruhl H, Maschmeyer G, Lipp T, Nowrousian MR, Burkert M, Gerecke D, Pralle H, Muller U, Lunscken C, Fulle H, Ho AD, Kuchler R, Busch FW, Schneider W, Gorg C, Emmerich B, Braumann D, Vaupel HA, Paleske A, Bartels H, Neiss A, Messerer D. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988. 71:123–131.

17. Stock W, Yu D, Johnson J, Bloomfield CD, Stone RM, Kolitz JE, Wetzler M, Powell BL, Vardiman JW, Larson RA. Intensified daunorubicin during induction and post-remission therapy of adult acute lymphoblastic leukemia (ALL): results of CALGB 19802. Blood. 2003. 102:379a.
18. Hallbook H, Simonsson B, Ahlgren T, Bjorkholm M, Carneskog J, Grimfors G, Hast R, Karlsson K, Kimby E, Lerner R, Linder O, Linderholm M, Lofvenberg E, Malm C, Nilsson PG, Paul C, Stenke L, Stockelberg D, Tidefelt U, Turesson I, Uden-Blome AM, Vilen L, Wahlin A, Winquist I, Smedmyr B. High-dose cytarabine in upfront therapy for adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2002. 118:748–754.
20. Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, Katz J, Yu A, Laver J, Ravindranath Y, Kurtzberg J, Desai S, Camitta B, Murphy SB. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999. 13:335–342.

21. Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, Lazarus HM, Franklin IM, Litzow MR, Ciobanu N, Prentice HG, Durrant J, Tallman MS, Goldstone AH. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005. 106:3760–3767.

22. Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002. 20:2464–2471.

23. Nagura E, Kimura K, Yamada K, Ota K, Maekawa T, Takaku F, Uchino H, Masaoka T, Amaki I, Kawashima K, Ohno R, Nomura T, Hattori J, Kawamura S, Shibata A, Shirakawa S, Hamajima N. Nationwide randomized comparative study of doxorubicin, vincristine and prednisolone combination therapy with and without L-asparaginase for adult acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 1994. 33:359–365.

24. Takeuchi J, Kyo T, Naito K, Sao H, Takahashi M, Miyawaki S, Kuriyama K, Ohtake S, Yagasaki F, Murakami H, Asou N, Ino T, Okamoto T, Usui N, Nishimura M, Shinagawa K, Fukushima T, Taguchi H, Morii T, Mizuta S, Akiyama H, Nakamura Y, Ohshima T, Ohno R. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG-ALL93 study. Leukemia. 2002. 16:1259–1266.

25. Wetzler M, Sanford BL, Kurtzberg J, DeOliveira D, Frankel SR, Powell BL, Kolitz JE, Bloomfield CD, Larson RA. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood. 2007. 109:4164–4167.

26. Dufourg MN, Landman-Parker J, Auclerc MF, Schmitt C, Perel Y, Michel G, Levy P, Couillault G, Gandemer V, Tabone MD, Demeocq F, Vannier JP, Leblanc T, Leverger G, Baruchel A. Age and high-dose methotrexate are associated to clinical acute encephalopathy in FRALLE 93 trial for acute lymphoblastic leukemia in children. Leukemia. 2007. 21:238–247.

27. Quinn CT, Kamen BA. A biochemical perspective of methotrexate neurotoxicity with insight on nonfolate rescue modalities. J Investig Med. 1996. 44:522–530.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download