Abstract
Neointimal hyperplasia causes vascular stenosis and subsequent thrombosis, which result in vascular access failure in patients undergoing hemodialysis. Interleukin-10 (IL-10) and tumour necrosis factor-α (TNF-α ) are involved in this inflammatory process. The aim of this study was to investigate the relationship between vascular accessm failure and various inflammatory markers including the genetic polymorphisms of IL-10 and TNF-α . Seventy-five patients on hemodialysis with an arteriovenous fistula in place or an artificial graft (18 with vascular access failure and 82 without failure) and 98 healthy individuals were genotyped for IL-10 and TNF-α single nucleotide polymorphisms. Clinical and laboratory data including serum IL-10 and TNF-α levels were compared. Stimulated IL-10 levels, from in vitro incubation of blood with lipopolysaccharide, were also obtained and compared. Female gender, hypoproteinemia, and hypertriglyceridemia were associated with vascular access failure. The basal TNF-α level was significantly higher in patients with access failure. The distribution of IL-10 and TNF-α genotype did not differ among patients with or without access failure. This study could not demonstrate a relationship between genetic polymorphisms and vascular access failure. However, an altered immune response and inflammation might contribute to vascular access failure.
Vascular access failure, the most frequent cause of morbidity and hospitalization in patients undergoing hemodialysis, is primarily due to vascular stenosis, which predisposes to thrombosis and subsequently leading to access obstruction. Neointimal hyperplasia (NIH) is believed to be the predominant cause of vascular stenosis of both the arteriovenous fistula and the polytetrafluoroethylene (PTFE) graft (1, 2). The pathophysiology of NIH consists of an aberrant wound healing process characterized by vascular smooth muscle cell (VSMC) migration, adherence, proliferation, and extracellular matrix deposition. The altered VSMC response is mediated in part by cytokines and growth factors. Tumor necrosis factor-α (TNF-α) stimulates the synthesis of other pro-inflammatory cytokines and adhesion molecules; it has a chemotactic activity for monocytes and stimulates migration and proliferation of VSMC. Interleukin-10 (IL-10) exerts an anti-inflammatory activity, which inhibits inflammatory cytokines such as TNF-α and inactivates inflammatory cells.
Inflammatory cytokine genes, or their promoter regions, have single nucleotide polymorphisms that influence the rate and magnitude of cytokine production. Many cytokine gene polymorphisms have been reported to be associated with a predisposition to develop a variety of inflammatory diseases (3, 4). The transforming growth factor-β1 (TGF-β1) gene polymorphisms were reported to influence the risk for arteriovenous fistula failure in patients undergoing hemodialysis (5). The TNF-α -308 gene polymorphism has been reported to be associated with PTFE graft failure (3).
In this study, we examined the relationship between various inflammatory markers and vascular access failure. We also evaluated the influence of TNF-α and IL-10 genetic polymorphisms on the development of vascular access failure.
From January to December 2005, 100 patients on maintenance hemodialysis at the Korea University Anam Hospital were selected for this cross-sectional study. Ninety-eight healthy individuals were recruited from the Medical Examination Center of Korea University Anam Hospital to serve as control. Informed consent was obtained in accordance with the guidelines set forth by the Declaration of Helsinki. Clinical information, laboratory data, and history of vascular accesses of study patients were assessed by a chart review. Vascular access failure was defined as the need for any angioplastic or surgical intervention to correct poorly or non-functioning fistula or graft.
Genomic DNA was prepared from peripheral blood mononuclear cells from patients and from healthy controls using the QIAmp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's specifications. Briefly, 1 mL of whole blood was collected from a patient in a heparinized tube, digested with proteinase-K, and submitted to a silica gel QIAmp spin column. The blood column was washed with appropriated buffers, and the total DNA was eluted with 200 µL of elution buffer. The total DNA recovered from each sample was quantified and assessed for purity by spectrophotometric analysis at 260 and 280 nm.
Cytokine genotyping for IL-10 (-1,082 G/A) and TNF-α (-308 G/A) was performed using the amplification refractory mutational system method (ARMS-PCR). Briefly, 0.1 µg of total DNA was amplified by PCR in a 10 µL reaction mixture containing 1 × AS reaction buffer, 200 µM deoxynucleotide triphosphate (dNTP), 1.5 mM MgCl2, 8.5% sucrose, 0.25 units ThermoprimePLUS DNA polymerase, 5 µM specific primer mixture, and 1 µM internal control primer mixture. The primer sets for TNF-α -308 PCR were generic primer 5'-TCTCGGTTTCTTCTCCATCG-3', primer G 5'-ATAGGTTTTGAGGGGCATGG-3', and primer A 5'-AATAGGTTTTGAGGGGCATGA-3'. The primer sets for IL-10 -1,082 PCR are generic primer 5'-CAGTGCCAACTGAGAATTTGG-3', primer G 5'-CTACTAAGGCTTCTTTGGGAG-3', and primer A 5'-ACTACTAAGGCTTCTTTGGGAA-3'.
A peripheral blood sample was obtained in a heparinized bottle prior to hemodialysis and was divided into two wells. One µg/mL of lipopolysaccharide (LPS E. coli, Sigma, St Louis, U.S.A.) was added to one well. Samples and controls without LPS were incubated at 37℃, 5% CO2 for 24 hr and spun for 15 min, and the serum supernatant aliquots were stored at -20℃ until analyzed. The serum IL-10 level was measured by the quantitative sandwich enzyme- linked immunosorbent assay (Quantikine, RD systems, Minneapolis, U.S.A.) in accordance with the manufacturer's instructions.
TNF-α was studied on blood samples taken before hemodialysis and allowed to clot; the samples were then spun for 15 min, and the serum supernatant aliquots were frozen at -20℃. The serum TNF-α level was measured by the same method as above.
Data management and statistical analysis were done using the SPSS software. The χ2-test was used to compare the genotype frequencies between healthy control and patient groups. The nonparametric Mann-Whitney-U test was used to compare continuous data including serum cytokine levels between patients with or without vascular access failure. The Kruskal-Wallis test was used for comparisons between healthy control and two patient groups. A p value <0.05 was considered as significant.
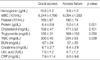
Eighteen among 100 patients had vascular access failure. Comparisons of baseline characteristics among the patients are shown in Table 1. The patients on hemodialysis were much older than the healthy control group. The proportion of female and the proportion of PTFE graft were higher in the vascular access failure group than the good functioning access group. The prevalence of diabetes and hypertension, and duration of hemodialysis and vascular access were not different between patients with or without vascular access failure. Hypoproteinemia and hypertriglyceridemia were much more frequent among the patients with vascular access failure (Table 2).
The distribution of genotypes were 40% AA, 48% AG, 12% GG for -1,082 IL-10, and 8% AA, 40% AG, 52% GG for -308 TNF-α. There was no difference in genotype frequencies between the patients with vascular access failure and those with good functioning access (Fig. 1).
Although the patients on hemodialysis produced a higher level of LPS-stimulated IL-10 than healthy controls, no significant differences were revealed among the patients with or without access failure. Patients genotyped as A negative at -1,082 IL-10 showed a higher level of LPS-stimulated IL-10 level, GG>AG>AA (Fig. 2). There was no significant difference in basal IL-10 levels.
Basal TNF-α was much higher in the vascular access failure group compared to the good functioning access group (Fig. 3). Patients with AG at -308 TNF-α exhibited a significantly higher level of basal TNF-α than GG or AA.
An aberrant wound healing process in response to chemical or mechanical injury is believed to explain the pathophysiology of NIH; VSMC proliferates and migrates into the intima of vessels, where they induce intimal expansion via extracellular matrix deposition. Although it is not clear whether the fibroproliferative response occurs by way of inflammatory pathways or whether the inflammation is secondary to other driving mechanisms, several studies have suggested that inflammatory cytokines play a critical role in NIH. TNF-α, a proximal inflammatory cytokine, stimulates the expression of adhesion molecules and other pro-inflammatory cytokines including platelet-derived growth factor (PDGF) and TGF-β 1, which are important mediators of VSMC proliferation and migration (6). The TNF-α level was shown to increase for several days prior to migration of VSMC into the intima, in balloon-injured rat aorta; the TNF-α antagonist-inhibited coronary artery NIH in cholesterol-fed rabbits after cardiac transplantation (7, 8). IL-10 exerts anti-inflammatory activities directed against the function of inflammatory cells, and inhibits the production of inflammatory cytokines, such as TNF-α , IL-1, and IL-8. IL-10 was shown to interfere with NIH, after balloon injury or stent implantation, in hypercholesterolemic rabbits, and potently abrogates the proliferative response to atherogenic mitogens (8, 9).
The critical role of TNF-α and IL-10 in NIH and the functional relevance of the single nucleotide polymorphism suggest a relationship between TNF-α and IL-10 gene polymorphisms and vascular access failure in patients on hemodialysis. Single nucleotide polymorphisms, located in the promoter regions of the TNF-α gene -308 and the IL-10 -1,082, have been found to differentially affect binding of nuclear transcription factors, transcriptional activity, and protein production (4, 10-12). Expression of the A allele in -308 TNF-α gene results in increased genetic transcription and subsequent production of TNF-α compared to the normal production by the genotype GG, and the presence of the A allele is associated with increased mortality in diabetic patients (12, 13). The TNF-α -308 gene polymorphism has been reported to be associated with PTFE graft failure (3). However, based on our results, the functionally relevant single nucleotide polymorphisms of the genes encoding TNF-α and IL-10 did not correlate with vascular access failure. These negative results should not be interpreted as an argument against the importance of inflammatory responses elicited in vascular access failure and the critical role of TNF-α and IL-10 as regulators. Instead, apparent lack of association may reflect the complexity of interactions underlying the NIH and subsequent vascular stenosis, and may also largely be attributable to the inadequate sample size available for our study. In fact, previous studies on the IL-10 -1,082 polymorphism have not always revealed a relationship with inflammatory disease. A relationship was not demonstrated in coronary artery restenosis after angioplasty (14), rheumatoid arth- ritis and chronic hepatitis (15, 16), development of cervical cancer (17), and prognosis of lymphoma and breast cancer (18, 19).
In addition to the small sample size, there were other limitations in this study. The patients examined in this study were all Koreans, and different results may be obtained with populations of other ethnic origins. The distribution of genetic polymorphisms of the IL-10 -1,082 and TNF-α -308 differ in Caucasian populations (20). We did not compare the diameter of the access vessels, an important factor for the development of vascular access failure (21). Lastly, we did not measure the stimulated TNF-α level.
The results showed that female gender, hypoproteinemia, and hypertriglyceridemia were associated with vascular access failure. This is in agreement with previous reports (21, 22). Female patients may have vessels of smaller diameter. Ernandez et al. reported that female gender appears to be an independent risk factor for early failure of AVF when adjusted for initial artery diameter (21). Hypoproteinemia, as a result of inflammation, might be related with vascular access failure. Inflammation decreases the synthesis of protein in liver, and is associated with a greater fractional catabolic rate and, when extreme, increases the transfer of albumin out of the vascular compartment. A vicious cascade of events ensues in which inflammation induces anorexia and reduces the effective use of dietary protein and energy intake and augments catabolism of protein. De Marchi et al. reported that a high total cholesterol/HDL cholesterol ratio and hypertriglyceridemia are powerful risk indicators for fistula obstruction of patients on hemodialysis (22).
In conclusion, this cross-sectional study did not reveal a relationship between vascular access failure and single nucleotide polymorphisms of IL-10 -1,082 and TNF-α -308. However, female gender, hypoproteinemia, and hypertriglyceridemia could be risk factors for the development of access failure, and the imbalance of cytokine networks including TNF-α from uremia could have influenced the development of access failure. Further prospective studies involving a large study population should follow to inrolentify factors that are useful in detection of risk factors for access failure to improve the management of patients on hemodialysis.
Figures and Tables
Fig. 1
Comparison between percentage frequencies of interleukin-10 and tumour necrosis factor-α genotypes in healthy controls, good functioning access groups and access failure group.

Fig. 2
Comparison between interleukin-10 single nucleotide polymorphisms in in vitro production of interleukin-10 protein in peripheral blood mononuclear cells after stimulating with lipopolysaccharide.
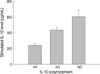
Fig. 3
Comparison of the serum tumour necrosis factor-α level between the good functioning access group and the access failure group.
References
1. Rekhter M, Nicholls S, Ferguson M, Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993. 13:609–617.

2. Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001. 59:2325–2334.

3. Ram S, Bass K, Abreo K, Baier RJ, Kruger TE. Tumor necrosis factor-alpha -308 gene polymorphism is associated with synthetic hemodialysis graft failure. J Investig Med. 2003. 51:19–26.
4. Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, Tsunoda T, Sato H, Hori M, Nakamura Y, Tanaka T. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet. 2002. 32:650–654.
5. Heine GH, Ulrich C, Sester U, Sester M, Kohler H, Girndt M. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int. 2003. 64:1101–1107.
6. Lemson MS, Tordoir JH, Daemen MJ, Kitslaar PJ. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg. 2000. 19:336–350.

7. Rus HG, Niculescu F, Vlaicu R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis. 1991. 89:247–254.

8. Clausell N, Molossi S, Sett S, Rabinovitch M. In vivo blockade of tumor necrosis factor-alpha in cholesterol-fed rabbits after cardiac transplant inhibits acute coronary artery neointimal formation. Circulation. 1994. 89:2768–2779.

9. Feldman LJ, Aguirre L, Ziol M, Bridou JP, Nevo N, Michel JB, Steg PG. Interleukin-10 inhibits intimal hyperplasia after angioplasty or stent implantation in hypercholesterolemic rabbits. Circulation. 2000. 101:908–916.

10. Rees LE, Wood NA, Gillespie KM, Lai KN, Gaston K, Mathieson PW. The interleukin-10-1082 G/A polymorphism: allele frequency in different populations and functional significance. Cell Mol Life Sci. 2002. 59:560–569.
11. Rosenwasser LJ, Borish L. Genetics of atopy and asthma: the rationale behind promoter-based candidate gene studies (IL-4 and IL-10). Am J Respir Crit Care Med. 1997. 156:S152–S155.

12. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997. 94:3195–3199.
13. Haukim N, Bidwell JL, Smith AJ, Keen LJ, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D'Alfonso S. Cytokine gene polymorphism in human disease: on-line databases, supplement 2. Genes Immun. 2002. 3:313–330.

14. Koch W, Tiroch K, von Beckerath N, Schomig A, Kastrati A. Tumor necrosis factor-alpha, lymphotoxin-alpha, and interleukin-10 gene polymorphisms and restenosis after coronary artery stenting. Cytokine. 2003. 24:161–171.
15. Pawlik A, Kurzawski M, Szklarz BG, Herczynska M, Drozdzik M. Interleukin-10 promoter polymorphism in patients with rheumatoid arthritis. Clin Rheumatol. 2005. 24:480–484.

16. Abbott WG, Rigopoulou E, Haigh P, Cooksley H, Mullerova I, Novelli M, Winstanley A, Williams R, Naoumov NV. Single nucleotide polymorphisms in the interferon-gamma and interleukin-10 genes do not influence chronic hepatitis C severity or T-cell reactivity to hepatitis C virus. Liver Int. 2004. 24:90–97.

17. Zoodsma M, Nolte IM, Schipper M, Oosterom E, van der Steege G, de Vries EG, Te Meerman GJ, van der Zee AG. Interleukin-10 and Fas polymorphisms and susceptibility for (pre) neoplastic cervical disease. Int J Gynecol Cancer. 2005. 15:Suppl 3. 282–290.
18. Berglund M, Thunberg U, Roos G, Rosenquist R, Enblad G. The interleukin-10 gene promoter polymorphism (-1082) does not correlate with clinical outcome in diffuse large B-cell lymphoma. Blood. 2005. 105:4894–4895.

19. Wu JM, Bensen-Kennedy D, Miura Y, Thoburn CJ, Armstrong D, Vogelsang GB, Hess AD. The effects of interleukin 10 and interferon gamma cytokine gene polymorphisms on survival after autologous bone marrow transplantation for patients with breast cancer. Biol Blood Marrow Transplant. 2005. 11:455–464.
20. Reynard MP, Turner D, Navarrete CV. Allele frequencies of polymorphisms of the tumour necrosis factor-alpha, interleukin-10, interferon-gamma and interleukin-2 genes in a North European Caucasoid group from the UK. Eur J Immunogenet. 2000. 27:241–249.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download