Abstract
Monocyte chemoattractant protein-1 (MCP-1) is suggested to be involved in the progression of diabetic nephropathy. We investigated the association of the -2518 A/G polymorphism in the MCP-1 gene with progressive kidney failure in Korean patients with type 2 diabetes mellitus (DM). We investigated -2518 A/G polymorphism of the MCP-1 gene in type 2 DM patients with progressive kidney failure (n=112) compared with matched type 2 DM patients without nephropathy (diabetic control, n=112) and healthy controls (n=230). The overall genotypic distribution of -2518 A/G in the MCP-1 gene was not different in patients with type 2 DM compared to healthy controls. Although the genotype was not significantly different between the patients with kidney failure and the diabetic control (p=0.07), the A allele was more frequent in patients with kidney failure than in DM controls (42.0 vs. 32.1%, p=0.03). The carriage of A allele was significantly associated with kidney failure (68.8 vs. 54.5%, OR 1.84, 95% CI 1.07-3.18). In logistic regression analysis, carriage of A allele retained a significant association with diabetic kidney failure. Our result shows that the -2518 A allele of the MCP-1 gene is associated with kidney failure in Korean patients with type 2 DM.
Diabetic nephropathy is a common cause of end-stage renal disease (ESRD). The prevalence of diabetic nephropathy in ESRD has more than doubled in the past decade to reach 42.5% in Korea. It is also the major cause of morbidity and premature mortality in patients with type 2 diabetes mellitus (DM). The prevalence of progressive renal disease has generally been thought to be lower in type 2 DM (1). However, recent studies suggested that the cumulative incidence of renal complications in type 2 DM was similar to that seen in type 1 DM, which was about 25-30% at 20 yr after the diagnosis (2). However, this observation could not be applied to all patients with type 2 DM. Several population-based studies showed a prevalence of nephropathy of 5-10% at the time of diagnosis of type 2 DM (2), whereas only 20% of those with proteinuria finally developed ESRD (3).
High blood pressure, poor glycemic control, and albuminuria are well-known risk factors for the development or the progression of diabetic nephropathy, but these factors could not explain all of the inter-individual variabilities in the rate of progression to end-stage renal failure (2, 4). Recently, there were several reports that suggested that inflammation would have an important role in the pathogenesis of diabetic nephropathy in type 2 DM. Monocyte chemoattractant protein-1 (MCP-1) is a member of the famous chemokine family associated with various inflammatory diseases including glomerulonephritis, asthma, atherosclerosis, and Kawasaki disease (5-8).
MCP-1 is also a well known pathogenic mediator in the progression of diabetic nephropathy. The elevated MCP-1 in urine was demonstrated in type 2 DM patients with overt proteinuria. In addition, urinary MCP-1 level had a good correlation with the urinary albumin excretion (9).
The aim of the present study was to investigate the impact of the MCP-1 gene on diabetic kidney failure in Korean patients with type 2 DM. We investigated a well-known, -2518 G/A, polymorphism of the MCP-1 gene in type 2 DM patients with kidney failure compared with patients without nephropathy.
All consecutive patients with type 2 DM attending the renal replacement clinics at the Kyung Hee Medical Center, Seoul, Korea from 2000 to 2003 were screened. The diagnosis of type 2 DM was based on clinical characteristics that included: 1) no episodes of ketoacidosis; 2) diagnosis of DM after the age of 40 yr; 3) treated by diet alone, or in combination with oral hypoglycemic agents or fasting serum C-peptide values greater than 1.0 ng/mL (0.333 nM/L) in patients administered with insulin.
Those who satisfied the following criteria were recruited in the progressive diabetic kidney failure group: 1) history of type 2 DM precedes the history of nephropathy or chronic kidney disease; 2) normal kidney sizes by renal ultrasonography at the time of diagnosis of kidney failure; 3) retinopathy, defined as both background and proliferative retinopathies; 4) rule out other causes of end-stage renal failure; 5) kidney failure within 20 yr from the time of diagnosis of DM. Total of 112 patients fulfilled these criteria. A control group was selected from five thousand patients with type 2 DM attending the diabetic clinics at the same hospital. We examined the records of our patients who had normal renal function and no evidence of nephropathy. Age and sex matched 112 patients with type 2 DM were recruited as a control group, who satisfied the following criteria; 1) a follow-up duration longer than 10 yr since the onset of type 2 DM; 2) normal glomerular filtration rate (creatinine clearance ≥90 mL/min/m2); and 3) no evidence of proteinuria or microalbuminuria.
Finally, 230 unrelated healthy blood donors who underwent routine health checkup were also included to rule out the genetic effect of MCP-1 polymorphism on the development of type 2 DM.
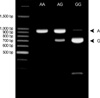
Venous blood samples were collected, and genomic DNA was isolated from peripheral blood. Polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) was performed to detect genotypes in the DNA sequence variants of the MCP-1 promoter. The forward primer was 5'-CCG AGA TGT TCC CAG CAC AG-3', and the reverse primer was 5'-CTG CTT TGC TTG TG CCT CTT-3'. DNA samples were amplified by PCR under the following conditions: 32 cycles of 45 sec at 94℃, 45 sec at 61℃, and 45 sec at 72℃. The extension time for the last cycle was set at 7 min. The PCR products were digested by the corresponding restriction enzyme PvuII (New England BioLab, Beverly, MA, U.S.A.) at 37℃. Digested products were separated on 1% agarose gels stained with ethidium bromide. Samples exhibiting 929-bp band were assigned as A/A, samples revealing two bands of 707 and 222 bp were typed as G/G, and samples illustrating three bands of 929, 707 and 222 bp were assigned as A/G (Fig. 1).
The allele frequency was calculated as the number of occurrences of the test allele in the population divided by the total number of alleles. The carriage rate was calculated as the number of individuals carrying at least one copy of the test allele divided by the total number of individuals.
Differences in proportions between patients with diabetic kidney failure and controls were compared by chi-square. The probability was considered significant at p<0.05. Odds ratio (OR) and 95% confidence intervals (CI) were calculated as estimates of the relative risks. Logistic regression analysis was used for the adjustment of other clinical variables. The SPSS statistical package (SPSS Inc., Chicago, U.S.A.) was used for statistical analysis.
Baseline characteristic data of subjects investigated in the present study are summarized in Table 1. This Table shows that the sex ratio and age did not differ significantly and were well matched between the progressive kidney failure group and the diabetic control group. The duration of diabetes was 16.9±5.6 yr in DM controls and 15.6±6.2 yr in the progressive kidney failure group. However, significant differences were found with respect to serum levels of creatinine and triglyceride and the presence of hypertension and retinopathy (Table 1).
The genotype and allele frequencies of the -2518G A/G polymorphism are shown in Table 2. The balance of heterozygotes and homozygotes conformed to the Hardy-Weinberg expectations, and there was no significant sex difference.
The overall genotype distribution of -2518 A/G in the MCP-1 gene was not significantly different in patients with type 2 DM compared to healthy controls (Table 2). However, in contrast to frequent A allele carriage in the Caucasian, G allele was more frequent in Korean patients with type 2 DM as a whole population.
The genotype distribution was not significantly different between the patients with kidney failure and the diabetic controls (A/A 15.1%, G/A 53.6%, G/G 31.3% vs. A/A 9.8 %, G/A 44.7%, G/G 45.5%, p=0.07). However, the -2518 A allele was significantly more frequent in patients with kidney failure than in DM controls (42.0% vs. 32.1%, p=0.03).
The carriage rate of -2518 A allele was 68.8% in the kidney failure group and 54.5% in DM controls. Relative to DM controls carrying no A allele, the risk of kidney failure in those carrying A allele was significantly elevated (odds ratio 1.84, 95% CI 1.07-3.18).
Among the clinical factors for diabetic kidney failure, the presence of hypertension revealed significant association with diabetic kidney failure as well as the -2518 A allele carriage in the MCP-1 gene. The odds ratio for those with hypertension was 9.49 (95% CI, 4.02-22.44). Through the logistic regression analysis including risk factors such as sex, age, hypertension, and poor glycemic control, the carriage of A allele retained still significant association with the risk of kidney failure (Table 3).
In the present study, we have investigated whether the specific polymorphisms in the MCP-1 gene was associated with kidney failure in Korean patients with type 2 DM, using a case-control study design. This study demonstrates that in contrast to frequent A allele carriage in the Caucasian, G allele carriage is more frequent in Korean population and A allele carriage was significantly associated with progressive kidney failure in Korean patients with type 2 DM. Because there is no difference in the frequency of A allele between healthy controls and patients with DM, A allele carriage was suggested to be related with the development of diabetic kidney failure rather than the occurrence of DM itself.
Recent lines of evidence showed that genetic predisposition affected the hyperglycemia-induced nephrotoxicity in patients with type 2 DM as well as type 1 DM (10). The polymorphisms in the ACE gene and several genes engaged in the renin-angiotensin-aldosterone axis were highlighted (11). Other candidate gene approaches were performed including endothelial nitric oxide synthetase, apolipoprotein E, heparan sulfate proteoglycan, and glycoprotein PC-1 (12-14).
There have been several reports, that suggested that inflammation would have an important role in the pathogenesis of diabetic kidney failure in type 2 DM. Chronic subclinical inflammation is an essential component of insulin resistance syndrome (15). Several studies showed that C-reactive protein, fibrinogen, interleukin-1, interleukin-6, and tumor necrosis factor- were increased in patients with type 2 DM (16).
Moreover, these inflammatory parameters were related with some of diabetic complications such as atherosclerosis, retinopathy, and nephropathy. Navarro et al. showed that urine albumin excretion was closely related with the inflammatory parameter such as TNF-α in urine, not in serum (17).
Monocyte chemoattractant protein-1 (MCP-1) was also a well-known pathogenic mediator in the progression of diabetic kidney failure. Increased glomerular expression of MCP-1 has been shown in several glomerular diseases (18). Upregulation of MCP-1 may be a common pathway involved in the progressive tubulointerstifial damage in diabetic kidney failure as well as in other inflammatory renal diseases (19).
Recently, several studies concerning the -2518 A/G polymorphism of the MCP-1 gene have been accomplished because the -2518 A/G polymorphism of MCP-1 may be associated with its gene expression. Rovin et al. reported that the G allele produced more MCP-1 in interleukin-1-stimulated peripheral mononuclear cells from healthy people and that the -2518 G frequency is over-represented in Asian and Mexican populations compared to a Caucasian population (20).
In a series of studies, G allele has generally been regarded to be associated with inflammation and also regarded as a somewhat negative predictor in several diseases such as Kawasaki disease, coronary artery disease, asthma, and lupus nephritis (5-8).
On the other hand, Simeoni et al. showed that in a large Caucasian cohort, the presence of the -2518 G allele was associated with decreased plasma MCP-1, decreased prevalence of insulin resistance, and decreased risk of DM (21). Kim et al. also reported that urinary excretion of MCP-1 was greater in AA homozygotes than in other genotypes in Korean patients with lupus nephritis (22).
Our study showed that the carriage of -2518 A allele in the MCP-1 gene was associated with the susceptibility of kidney failure in patients with type 2 DM. The association between the MCP-1 gene and diabetic nephropathy has not yet been reported in patients with type 2 DM. However, this association is in agreement with previous association studies, which suggested -2518 A in the MCP-1 gene might be associated with insulin resistance syndrome and type 2 DM (21) or nephritis in systemic lupus erythematosus (22).
Although Rovin et al. suggested that the -2518 G allele produced more MCP-1 in interleukin-1-stimulated peripheral blood mononuclear cells (20), the susceptibility to MCP-1 activation and the molecular mechanism by which the polymorphism regulates the MCP-1 gene transcription are still unclear. The distal regulatory region of the MCP-1 gene contains two NF-κB binding sites essential for cytokine-stimulated gene transcription, but this distal (-2518) A/G polymorphism did not alter the sequence of the NF-κB sites (23).
Moreover, previous association studies showed inconsistent results according to their race or disease phenotypes. We speculated that the inconsistent results from the previous association studies might be population-dependent. In the present study, we selected two clinically distinct groups; a progressive kidney failure group within 20 yr and a control group with no evidence of nephropathy in a relatively prolonged follow-up period.
Our result suggests that MCP-1 might play an important role in the progression of diabetic nephropathy. As shown in our study, high blood pressure is a well-known risk factor for the progression of diabetic nephropathy. Recently, arterial hypertension is also known to be associated with inflammation of the endothelium as an effect of the upregulation of functional molecules, including adhesion molecules and chemokines. In our study, the genotype of the MCP-1 gene was not associated with the presence of hypertension in patients with type 2 DM, whether they had kidney failure or not. As expected, the development of hypertension seems to be multifactorial event, especially in patients with type 2 DM.
Potential limitations of this study lie in the limited evaluations of other risk factors such as prolonged history of glycemic control, long-term effect of blood pressure or treatment with ACE inhibitor, which play an important role in the progression of diabetic nephropathy. A longitudinal study including appropriately adjusted clinical variables would be also helpful. Second, the polymorphic loci may be in linkage disequilibrium with an unidentified locus that is actually associated with kidney failure in type 2 DM. Measurement of the extent of linkage disequilibrium and identification of the haplotype at risk may be helpful.
Figures and Tables
Fig. 1
Representative 1% agarose gel stained with ethidium bromide and photographed under ultraviolet transillumination after PCR and digestion by Pvu II for MCP-1 genotyping. The upper band of 929 bp is the A allele and the lower band of 707 bp is the G allele (arrowheads). The A/A type is shown as a single upper band, the G/G type as a single lower band, and the G/A type as double bands.
References
1. Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989. 321:1074–1079.

2. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999. 341:1127–1133.

4. Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001. 59:702–709.

5. Szalai C, Kozma GT, Nagy A, Bojszko A, Krikovszky D, Szabo T, Falus A. Polymorphism in the gene regulatory region of MCP-1 is associated with asthma susceptibility and severity. J Allergy Clin Immunol. 2001. 108:375–381.

6. Szalai C, Duba J, Prohaszka Z, Kalina A, Szabo T, Nagy B, Horvath L, Csaszar A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1-2518 G/G genotype in CAD patients. Atherosclerosis. 2001. 158:233–239.
7. Jibiki T, Terai M, Shima M, Ogawa A, Hamada H, Kanazawa M, Yamamoto S, Oana S, Kohno Y. Monocyte chemoattractant protein 1 gene regulatory region polymorphism and serum levels of mono cyte chemoattractant protein 1 in Japanese patients with Kawasaki disease. Arthritis Rheum. 2001. 44:2211–2212.
8. Tucci M, Barnes EV, Sobel ES, Croker BP, Segal MS, Reeves WH, Richards HB. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004. 50:1842–1849.

9. Morii T, Fujita H, Narita T, Koshimura J, Shimotomai T, Fujishima H, Yoshioka N, Imai H, Kakei M, Ito S. Increased urinary excretion of monocyte chemoattractant protein-1 in proteinuric renal diseases. Ren Fail. 2003. 25:439–444.

10. Iyengar SK, Fox KA, Schachere M, Manzoor F, Slaughter ME, Covic AM, Orloff SM, Hayden PS, Olson JM, Schelling JR, Sedor JR. Linkage analysis of candidate loci for end-stage renal disease due to diabetic nephropathy. J Am Soc Nephrol. 2003. 14:7 Suppl 2. S195–S201.

11. Lovati E, Richard A, Frey BM, Frey FJ, Ferrari P. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int. 2001. 60:46–54.

12. Shin Shin Y, Baek SH, Chang KY, Park CW, Yang CW, Jin DC, Kim YS, Chang YS, Bang BK. Relations between eNOS Glu298Asp polymorphism and progression of diabetic nephropathy. Diabetes Res Clin Pract. 2004. 65:257–265.

14. Liu L, Xiang K, Zheng T, Zhang R, Li M, Li J. Co-inheritance of specific genotypes of HSPG and ApoE gene increases risk of type 2 diabetic nephropathy. Mol Cell Biochem. 2003. 254:353–358.
15. Festa A, D'Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000. 102:42–47.
16. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997. 40:1286–1292.

17. Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003. 42:53–61.

18. Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994. 5:1273–1287.

19. Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000. 58:1492–1499.

20. Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999. 259:344–348.

21. Simeoni E, Hoffmann MM, Winkelmann BR, Ruiz J, Fleury S, Boehm BO, Marz W, Vassalli G. Association between the A-2518G polymorphism in the monocyte chemoattractant protein-1 gene and insulin resistance and Type 2 diabetes mellitus. Diabetologia. 2004. 47:1574–1580.

22. Kim HL, Lee DS, Yang SH, Lim CS, Chung JH, Kim S, Lee JS, Kim YS. The polymorphism of monocyte chemoattractant protein-1 is associated with the renal disease of SLE. Am J Kidney Dis. 2002. 40:1146–1152.

23. Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004. 19:2505–2512.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download