Abstract
The clinicopathological findings in previous studies concerning food protein-induced proctocolitis (FPIPC) are quite diverse in terms of results and conclusions. The aim of this study was to suggest advanced clinicopathological diagnostic criteria that facilitate the early confirmation of FPIPC. Data of 38 FPIPC patients, who had received sigmoidoscopy and biopsy, was analyzed. Microscopic findings were compared with observations of previous studies. Feeding at onset of bleeding was exclusively breast-fed (94.7%) and formula-fed or mixed-fed (5.3%). Endoscopic abnormalities were observed in all patients; nodular hyperplasias with circumscribed and/or central pit-like erosions in 94.7% and erythema in 5.3%. Histopathological findings were; lymphoid aggregates in 94.7%, eosinophils in lamina propria of ≥60 cells/10 HPF in 97.4% and of >20 cells/HPF in 63.2%, epithelial or muscularis mucosa eosinophil infiltration in 97.4%, and crypt abscess in 2.6%. The majority of FPIPC patients are exclusively breast-fed and nodular hyperplasias with erosions may be a disease specific endoscopic finding. Histologic diagnosis of FPIPC is compatible with eosinophils in the lamina propria of ≥60 cells/10 high power fields; however, >20 cells/HPF is not an appropriate diagnostic criterion.
Therapy for infants with food protein-induced proctocolitis (FPIPC) is based on the elimination of presumed triggering antigens (1, 2). However, maternal diet manipulation or change to a protein hydrolysate or amino acid formula causes parents to view a child as sick, they may continue unnecessary dietary restrictions into later childhood (3). In addition, parents and family may be anxious or depressed due to the high costs incurred and possible social isolation.
In the survey of North American Society for Pediatric Gastroenterology, Hepatology and Nutrition members, 84% indicated they would empirically change the diet of an infant with rectal bleeding to treat presumed FPIPC. However, in a Cincinnati study population, only 64% infants with rectal bleeding had FPIPC (4). Hemorrhagic colitis in newborn babies is a heterogeneous disease group, in where dietary protein intolerance may account for some cases (5, 6).
Therefore, clarified clinicopathological diagnostic criteria are mandatory for the early recognition and conformation of FPIPC, and could be used to guide cost effective and systematic treatment. However, the clinical, endoscopic, and histopathological findings in previous studies (7-12) associated with FPIPC are quite different in terms of results and conclusions, and also with our clinical experiences of FPIPC patients.
Thus, the aim of this study was to identify disease specific clinical, endoscopic, and histopathological findings, to aid the effective differential diagnosis of rectal bleeding diseases in neonates and early infants, and to suggest advanced diagnostic criteria to facilitate the early confirmation of FPIPC.
We analyzed the data of 38 FPIPC patients (female 15, male 23), who underwent sigmoidoscopy and biopsy, among 51 infants with rectal blood between March 2003 and July 2005. Patients were recruited from the outpatient and inpatient units at the Dongsan Medical Center, Keimyung University School of Medicine. Informed consent was obtained from the parents of all children who underwent sigmoidoscopy and biopsy. Study procedures were approved by the Keimyung University Institutional Review Board. We investigated age at symptom onset and diagnosis, feeding at onset of bleeding, anemia for age, serum albumin level, endoscopic findings, and histopathologies in the biopsy specimens of 38 FPIPC patients. Sigmoidoscopic procedures were performed at first visit using a GIF N230 or GIF Q260 (Olympus®, Japan) without conscious sedation at <6 months old, and with conscious sedation (midazolam; Dormicum®, Korea Roshe, Korea) at ≥6 months old. Patients were in a feeding state without nothing per oral and colons were gently cleansed by two successive half-isotonic saline water enemas (3-5 mL/kg) before the procedure. Endoscopy was used to explore to the splenic flexure and the gross appearances and locations of lesions were recorded. Endoscopic biopsies were performed in the sigmoid portion (about 8 cm from the anal verge) and 3 specimens were obtained from each of the 38 patients (114 total specimens).
FPIPC was diagnosed based on clinical findings using a modification of the diagnostic guidelines suggested by previous reports (4, 8-10, 13). A diagnosis of FPIPC was defined when three criteria were satisfied; 1) small and bright red rectal bleeding with mucus in a relatively healthy neonate or infant, 2) no other rectal bleeding causes (anal fissure, polyp, bleeding diathesis, infection, necrotizing enterocolitis, pseudomembranous colitis, Hirschsprung disease, or some other surgical abdominal condition) or systemic symptoms (febrile illness, irritability, failure to gain weight, weight loss), and 3) the disappearance of rectal blood after a change of diet. In breast mothers, 5 highly allergenic foods (namely, dairy products, eggs, nuts and soybean, fish and shellfish, and wheat and buckwheat) were eliminated from the maternal diets for 5 days (14). Of 49 patients whose mother ate the eliminated diet, hematochezia disappeared in 36 (73.5%) patients. In formula feeding patients, a dietary change to protein hydrolysate formula was done. A change to protein hydrolysate formula (HA®, Maeil Dairy Industry Co., Korea) in two patients resulted in the disappearance of rectal blood in both. Therefore, we analyzed the data of 38 FPIPC patients.
Table 1 shows the sigmoidoscopic and histopathologic observations of FPIPC in the present study. Eight parameters of microscopic findings in 38 FPIPC patients were interpreted blindly from biopsy specimens by two pediatric pathologists without any clinical information. Results of the interpreted eight parameters were compared with the previous observations of Odze et al. (9, 13) and Machida et al. (10). Table 1 shows the number of patients, biopsy sites, and total specimen counts of the three FPIPC studies. The maximum number of eosinophils per high-power field (HPF) (×400) in the single most involved area of biopsy was recorded.
Fisher's exact test was used for the comparisons of sigmoidoscopic nodularity, histologically lymphoid aggregates, and maximum number of eosinophils in the lamina propria per HPF in three studies. Correlation analysis was used to determine the nature of the correlation between peripheral blood eosinophil count and maximum count of infiltrated eosinophils per HPF in single most infiltrated biopsy. Statistical significance was accepted for p values of <0.05. Data are presented as mean±SD.
In the 38 FPIPC patients, feeding at the onset of bleeding was exclusively breast-fed (94.7%) and formula-fed or mixed-fed (5.3%). Mean age at symptom onset was 10.3±4.7 (3-24) weeks and mean age at diagnosis was 18.2±9.7 (8-56) weeks. Anemia for age was noted in 2.5% and patient mean serum albumin was 4.1±0.4 (3.6-4.9) g/dL. Peripheral eosinophilia (>500/µL) was observed in 31.6% of the 38 patients.
Endoscopic abnormalities were observed in all 38 FPIPC patients. Rectosigmoid lesions were observed in all patients and extension into the descending colon was noted in 31.6%. Nodular hyperplasias with circumscribed (Fig. 1A) and/or central pit-like erosions (Fig. 1B) were observed in 94.7% (p=0.0001 vs. result of Odze et al. and p=0.0001 vs. that of Machida et al.) (Table 1). Nodular hyperplasia was prominent in an endoscopically deflated state (Fig. 1C). Erythematous mucosal lesions without nodular hyperplasia were noted in 5.3%.
Table 1 shows the eight microscopic findings of the present study and those of the other two reports (9, 10, 13). Pathologic abnormalities were observed in the 3 biopsy specimens as follows; a single specimen abnormality in 7.9% of 38 FPIPC patients, 2 specimen abnormalities in 13.2%, and 3 specimen abnormalities in 78.9%. Eosinophilic infiltration was observed mainly in the lower lamina propria (84.2%). Lymphoid aggregates were present in 94.7% (p=0.001 vs. results of Odze et al. and p=0.0001 vs. that of Machida et al.). Eosinophil infiltration was observed in the lamina propria ≥60 cells/10 HPF in 97.4% and >20 cells/HPF in 63.2% (p=0.0001 vs. result of Machida et al.). Epithelial or muscularis mucosa eosinophil infiltration ≥1 cell/HPF was noted in 97.4% and crypt abscess in 2.6%. Crypt architecture destruction was not observed in any FPIPC patient. Peripheral eosinophilia was not found to be correlated with the degree of histological eosinophilic infiltration.
The clinical diagnosis of FPIPC can be difficult. The most practical approach is a positive response to the elimination of a presumed diet (13). Although careful history taking detailing reactions to feeding may be helpful, the presence or absence of such features has not been shown to have any predictive value in terms of identifying infants with FPIPC (15). The onset of FPIPC may be acute but is more often insidious. Hence, it may be difficult to recognize adverse reactions that might result from a specific food (1). Moreover, no single laboratory or biochemical test is sensitive or specific enough to be of diagnostic usefulness in FPIPC (13). Sigmoidoscopy in conjunction with the evaluation of multiple mucosal biopsy specimens may be helpful for confirming a diagnosis, particularly in patients whose condition does not improve after starting an elimination diet protocol (13). However, the endoscopic and histopathologic results of previous studies (9, 10, 13) differ and may confuse the differential diagnosis with other rectal bleeding disease, and delay the early confirmation of FPIPC.
Originally described in exclusively breast-fed infants (7), FPIPC has been reported in subsequent studies in infants receiving cow's milk or soy formula (1). Our observations of consecutive FPIPC patients, in the present study, revealed that FPIPC might more commonly occur in infants exclusively breast-fed. Although about 45% of infants were exclusively breast fed in previous report (9), absolute breast feeding has been suggested in other studies (7, 11, 12, 16-19) to be closer to the pathogenesis of FPIPC, which is compatible with our present observation. Lake et al. (7) documented the offending effect of dietary proteins passed in the mother's milk to FPIPC patients. Dupont et al. (5) reported that in infants breast fed at onset of bleeding, spontaneous resolution of bleeding occurred after instituting cow's milk formula feeding. Laitinen et al. (19) recently reported that an increase in the concentrations of prostaglandin E2 and cysteinyl leukotrienes in the breast milk and the presence of mother's allergic disease reduced gross blood in the stools of a breast-fed infant, and thus, concluded that a combination of immunomodulatory factors may protect an infant from gross blood in the stools of breast-fed infants. We presume that the exclusive breast feeding observed in most FPIPC patients may be important for its differential diagnosis in neonates and infants with rectal bleeding, and in approaches aimed at identifying the immunologic mechanisms responsible for FPIPC.
Gross endoscopic findings in previous FPIPC associated reports (4, 9-13), included diffuse erythema, friability, nodular hyperplasia and/or ulcerations. Thus, Xanthakos et al. (4) suggested that endoscopic findings alone are unspecific and thus unhelpful for the diagnosis of FPIPC or for its differential diagnosis from other rectal bleeding diseases in neonates or early infants. However, the present study reveals that nodular hyperplasias with characteristic erosions may be specific findings of FPIPC. Rectal bleeding during the neonatal or infantile period is an alarming event and requires urgent hospital care and investigation (5). Therefore, these disease specific endoscopic features may be important in the differential diagnosis of rectal bleeding diseases in neonates and infants. Lymphoid nodular hyperplasia (LNH) has been described as a sign of food allergy in children (20, 21). However, several infants with LNH, but without histologic evidence of FPIPC, were reported to have shown spontaneous resolution of bleeding over a period of a few weeks with no diet change (4). LNH was also noted in inflammatory colitis patients or normal controls (8). We believe that LNH itself may be not a disease specific finding for FPIPC, but gross endoscopic findings, multiple nodular hyperplasia with a 'goose pimply appearance' (12) predominantly in the endoscopically deflated state with characteristic circumscribed and/or central pit-like erosions (Fig. 1), may be helpful for the early recognition of FPIPC and for the differential diagnosis of rectal bleeding diseases in this age group.
In terms of gross endoscopic findings, involvement of the rectosigmoid was universal, whereas extension into the descending colon was limited to 31.6%. Hence, the biopsy location performed in the sigmoid portion (8 cm from the anal verge) in the present study might be appropriate for the evaluation of FPIPC. Histopathological results at this location and specimen counts were satisfactory as compared with those of other studies (9, 10, 13) for the diagnosis and evaluation of FPIPC. We presume that descending colon biopsies may decrease the diagnostic effectiveness of histopathologic studies for FPIPC. Also, in the present study, histological lymphoid aggregates were observed in 94.7%, which is significantly higher than reported previously; in 55.0% in Odze et al. study (9, 13) (p=0.001) and 29.4% in Machida et al. report (10) (p=0.0001). This may be the result of biopsy site and specimen count differences and the high frequency of nodular hyperplasia observed in the present study. We suggest that, together with gross endoscopic findings, histological lymphoid aggregates observed in biopsy specimens of the sigmoid portion are important diagnostic clues of FPIPC and differential diagnostic points when considering other rectal bleeding diseases.
The prominence of eosinophilic infiltrates in the rectosigmoid mucosa is a hallmark of FPIPC and suggests an important pathogenic role for eosinophils. The guidelines suggested by Odze et al. (9, 13) and Machida et al. (10) are important for the histopathologic diagnosis of FPIPC. The major diagnostic guideline of Odze et al. concerns eosinophil infiltration in the lamina propria of ≥60 cells/10 HPF, whereas Machida et al. suggested eosinophil infiltration in lamina propria of >20 cells/HPF as a diagnostic criterion. However, these different levels of infiltrated eosinophil counts are confusing in histopathological diagnosis of FPIPC. This present study reveals that only 63.2% of patients met Machida's criterion. However, eosinophilic infiltration in the lamina propria of ≥60 cells/10 HPF was satisfied by 97.4% of patients in the present study. Thus, we suggest that Machida's diagnostic guideline may be inappropriate as a diagnostic criterion of FPIPC. Also, diagnostic criteria of eosinophil infiltration count and epithelial or muscularis mucosa eosinophil infiltration (≥1 cell/HPF) of Odze et al. were compatible with the findings of the present study, and may serve as important histological diagnostic criteria for differentiating FPIPC from infectious colitis (8). Microscopically, the normal architecture of the mucosa was preserved in all cases in the present study, which concurs with observations of Odze et al. This finding is also important observation in the differential diagnosis of FPIPC and other rectal bleeding diseases.
In the present study, peripheral eosinophilia was observed in 31.6%, but was not found to be correlated with the level of histological eosinophilic infiltration, which is compatible with Odze et al. (9, 13) and Machida et al. (10) observations. Anemia and serum hypoalbuminemia were reported in the other reports (7, 9, 10, 17, 18) to be associated with FPIPC. However, our results revealed only a patient with anemia for age and no serum hypoalbuminemia in all patients.
In summary, the majority of FPIPC patients were found to be exclusively breast-fed at the onset of bleeding. Moreover, nodular hyperplasias with circumscribed and/or central pitlike erosions may be specific endoscopic findings in FPIPC patients. Histopathological diagnostic guidelines of Odze et al. are strongly supportive of a clinical diagnosis of FPIPC, and are compatible with the majority of our observations. These clinical, endoscopic, and histopathological characteristic findings may facilitate the effective differential diagnosis of rectal bleeding diseases in neonates and infants and the early confirmation of FPIPC.
Figures and Tables
Fig. 1
Sigmoidoscopy shows nodular hyperplasias with circumscribed (A) and/or central pit-like erosions (B) in the patients with food protein-induced proctocolitis. Nodular hyperplasias are prominent in an endoscopically deflated state (C).
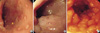
Table 1
Results of sigmoidoscopic observation and histopathology in food protein-induced proctocolitis compared with the previous observations of Odze et al. and Machida et al.*

*Odze et al. (Reference 9, 13) and Machida et al. (Reference 10). † p=0.0001 vs. Odze's and p=0.0001 vs. Machida's. ‡The maximum number of eosinophils per high-power field (HPF) (×400) in the single most involved biopsy was recorded. §p=0.001 vs. Odze's and p=0.0001 vs. Machida's. ∥p=0.0001 vs. Machida's. EOS, eosinophil; LP, lamina propria; n.d., no data; MM, muscularis mucosa.
References
1. Norwak-Wegrzyn A. Metcalfe DD, Sampson HA, Simon RA, editors. Food protein-induced enterocolitis, enteropathy, and proctocolitis. Food allergy: Adverse reactions to foods and food additives. 2003. 3rd ed. Baltimore: Blackwell Publishing, Inc;227–241.
2. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000. 106:346–349.
3. Forsyth BW, McCarthy PL, Leventhal JM. Problems of early infancy, formula changes, and mother's beliefs about their infants. J Pediatr. 1985. 106:1012–1017.

4. Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2005. 41:16–22.

5. Dupont C, Badoual J, Le Luyer B, Le Bourgeois C, Barbet JP, Voyer M. Rectosigmoidoscopic findings during isolated rectal bleeding in the neonate. J Pediatr Gastroenterol Nutr. 1987. 6:257–264.

6. Chong SK, Blackshaw AJ, Morson BC, Williams CB, Walker-Smith JA. Prospective study of colitis in infancy and early childhood. J Pediatr Gastroenterol Nutr. 1986. 5:352–358.

7. Lake AM, Whitington PF, Hamilton SR. Dietary protein-induced colitis in breast-fed infants. J Pediatr. 1982. 101:906–910.

8. Winter HS, Antonioli DA, Fukagawa N, Martial M, Goldman H. Allergy-related proctocolitis in infants: diagnostic usefulness of rectal biopsy. Mod Pathol. 1990. 3:5–10.
9. Odze RD, Bines J, Leichtner AM, Goldman H, Antonioli DA. Allergic proctocolitis in infants: a prospective clinicopathologic biopsy study. Hum Pathol. 1993. 24:668–674.

10. Machida HM, Catto-Smith AG, Gall DG, Trevenen C, Scott RB. Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr. 1994. 19:22–26.
11. Anveden-Hertzberg L, Finkel Y, Sandstedt B, Karpe B. Proctocolitis in exclusively breast-fed infants. Eur J Pediatr. 1996. 155:464–467.
12. Pumberger W, Pomberger G, Geissler W. Proctocolitis in breast fed infants: a constribution to differential diagnosis of hematochezia in early childhood. Postgrad Med J. 2001. 77:252–254.
13. Odze RD, Wershil BK, Leichtner AM, Antonioli DA. Allergic colitis in infants. J Pediatr. 1995. 126:163–170.

14. Choi SY, Park MH, Choi WJ, Kang U, Oh HK, Kam S, Hwang JB. Clinical features and the natural history of dietary protein induced proctocolitis: a study on the elimination of offending foods from the maternal diet. Korean J Pediatr Gastroenterol Nutr. 2005. 8:21–30.

15. Bock SA. Natural history of food sensitivity. J Allergy Clin Immunol. 1982. 69:173–177.
16. Lake AM. Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr. 2000. 30:Suppl. S58–S60.

17. Wilson NW, Self TW, Hamburger RW. Severe cow's milk-induced colitis in an exclusively breast-fed neonate. Clin Pediatr (Phila). 1990. 29:77–80.
18. Perisic VN, Filipovic D, Kokkai G. Allergic colitis and rectal bleeding in an exclusively breast-fed neonate. Acta Paediatr Scand. 1988. 77:163–164.
19. Laitinen K, Arvola T, Moilanen E, Lampi AM, Ruuska T, Isolauri E. Characterization of breast milk received by infants with gross blood in stools. Biol Neonate. 2005. 87:66–72.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download