Abstract
The bovine leukemia virus (BLV) is the causative agent of enzootic bovine leucosis. This study investigated the presence of the BLV in leukemia (179 acute lymphoblastic leukemia, 292 acute myeloid leukemia and 46 chronic myelogenous leukemia cases) and 162 lung cancer patients (139 adenocarcinoma, 23 squamous cell carcinoma) to determine if the BLV is a causative organism of leukemia and lung cancer in Koreans. A BLV infection was confirmed in human cells by PCR using a BLV-8 primer combination. All 517 cases of human leukemia and 162 lung cancer were negative for a PCR of the BLV proviral DNA. In conclusion, although meat has been imported from BLV endemic areas, the BLV infection does not appear to be the cause of human leukemia or lung cancer in Koreans. These results can be used as a control for further studies on the BLV in Koreans.
The bovine leukemia virus (BLV) infects and causes leukemia in cattle, and epidemiological studies have reported its infectivity and oncogenicity in humans (1). Some reports have confirmed that humans have been exposed to these viruses by demonstrating the presence of antibodies to the BLV in human sera (2). According to a study in the United States of America and Canada, more than 20% of cattle were found to have antibodies to this virus, and it is known that infected cows are mixed with non-infected cows, the latter constituting more than 70% of the herd (3). It is also known that workers handling infected meat in a food store have a threefold increased risk of contracting myeloid leukemia than the control subjects (4). In addition, workers who handle meat in a food store have a higher risk of lung cancer than the normal controls (5).
In Korea, a great deal of meat is imported from these countries, but there have been no concerns about the BLV or its genetic studies. Lung cancer is the most common type of cancer causing death in Korea. Increased consumption of imported meat contaminated with the BLV might be a reason of this increase in the lung cancer incidence in Korea.
Leukemia also accounts for one eighth of all cancer morbidities and mortalities in Korea. Therefore, it is essential to investigate the presence of the BLV in lung cancer and leukemic patients to determine the role of the BLV in cancers in Koreans.
A positive control cell line infected with the BLV BL-3.1, and a negative control cell line, BL-3, were purchased from ATCC (Manassas, VA, U.S.A). This study enrolled a total of 517 leukemia patients, comprising of 179 acute lymphoblastic leukemia patients, 292 acute myeloid leukemia patients and 46 chronic myelogenous leukemia cases patients in addition to 162 lung cancer cases (139 adenocarcinoma, 23 squamous cell carcinoma), who had been diagnosed at St. Mary's Hospital in Youido, Seoul, Korea.
A BLV positive cell line infected with the BLV, and a negative cell line were thawed in a water bath at 37℃, and were suspended in 5 mL of agar (BL-3: MEM-media, BL-3.1: RPMI 1640) containing 10% fetal bovine serum (FBS), then centrifuged for 5 min at 750 g. The sediment cells were suspended in 5 mL of agar containing 10% FBS, to a concentration of 4×105 cells/mL. These cells were then poured into a T-25 flask, and incubated in a 5% CO2 atmosphere at 37℃. These incubated cell lines were used for the experiment, and the leukemic cells were isolated from the bone marrow aspirates.
A Puregene DNA isolation kit (D-5500A; Gentra, Gentra Systems, Minneapolis, MN, U.S.A.) was used to extract the DNA from the cultured cell line and the leukemic cells. The DNA extraction from the leukemic cells was as follows: 800 µL of the bone marrow aspirates was mixed with 5 mL of a red blood cell lysis buffer, and incubated with intermittent mixing for 10 min at room temperature. After centrifugation at 2,000 g for 10 min at room temperature, the sediments were resuspended in 3 mL of the lysis buffer. One mL of a protein precipitation solution (10 M ammonium acetate) was added and mixed carefully. After centrifugation at 2,000 g for 10 min, the supernatant was transferred to a conical tube, and 3 mL of 100% isopropanol was added. The mixture then left in a 20℃ refrigerator for 20 min. The DNA was extracted after centrifugation at 2,000 g for 30 min at room temperature. The extracted DNA was purified by centrifugation with 3 mL of 70% ethanol under the above conditions.
The DNA from the paraffin blocks containing the lung cancer samples was extracted after removing the paraffin using xylene. After washing with ethanol, 400 µL of a cell lysis buffer and 5 µL of proteinase K were added and incubated overnight at 65℃. After precipitating the protein using a protein precipitation solution, the DNA was precipitated using ethanol, and the sediment was dried at room temperature.
The DNA hybridization solution was mixed with the dehydrated DNA obtained from the above procedure and used as a template after being left at 4℃ for 8 hr.
The PCR was performed using the genomic DNA extracted from the BLV infected cell line as a template by combining 2 or 3 primers in order to select the best primer. The pre-mix composition for the PCR in the 25 µL standards is as follows: 2.5 µL of 10× reaction buffer, 0.5 µL of 10 mM dNTPs, 1.0 µL of primer, 5.0 µL (50 ng/µL) of the template, 0.3 µL of Taq DNA polymerase (5 units/µL; SolGent, Daejeon, Korea) and 15.7 µL of distilled water. PCR was performed with the following cycles: cycle of pre-denaturation at 95℃ for 2 min, 35 cycles of amplification at 95℃ for 20 sec and 68℃ for 2 min, and 1 cycle of post-extension at 68℃ for 1 min. The sensitivity of the PCR amplification for the BLV detection was confirmed by follows: 40 cycles using the BLV-2 primer set (613 bps) and secondly 20 cycles under the same conditions. For the determination of PCR sensitivity, cloned PCR fragment amplified with BLV-2 by pCR 2.1 topo (Invitrogen, Carlsbad, CA, U.S.A.) was used as template. And copy number was fixed at 0.1-108 copies/reaction after measuring concentration of cloned plasmid by serial dilution. A DNA sequencer (ABI 3100, Applied Biosystems, Foster City, CA, U.S.A.) was used for the sequencing to confirm the amplified DNA.
In order to confirm the PCR amplification, the genomic DNA of the positive control cell line infected with BLV BL-3.1, and negative control cell line BL-3 were used as the templates of the positive and negative controls, respectively (Fig. 2). The primer set consisted of BLV-2, and BLV-8 was selected for the nested PCR (Fig. 3).
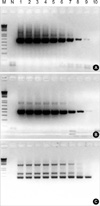
The sensitivity of the PCR amplification was 1 copy/reaction measured against the result of the nested PCR (Fig. 4).
The BLV envelope gene was confirmed by PCR product sequencing and from the result of a BLAST (basic local alignment search tool) search showing 414/418 (99%) identities.
Confirmation of the BLV proviral DNA in human leukemic cells and lung cancer was carried out by PCR using the BLV-8 primer combination. All 517 leukemia and 162 lung cancer specimens showed a negative PCR of the BLV with positive and negative control (Table 1).
The bovine leukemia virus (BLV) is an exogenous retrovirus that causes enzootic bovine leucosis. Under natural conditions, the disease occurs only in cattle, and an infection by the BLV can remain silent and clinically dormant in an aleukemic form. However, approximately one-third of infected cattle develop persistent lymphocytosis and 5-10% develop lymphoid tumors (7). The BLV has been reported to infect human cells in vitro, and cause tumors and erythroleukemia in primates (8, 9). Moreover, among workers in the meat department of retail food stores, a 3-fold increased risk of death has been observed for both myeloid leukemia and non-Hodgkin's lymphomas as well as lung cancer (10, 11). These results suggest the oncogenic capacity of the BLV in humans. The detection of the BLV proviral DNA sequence by PCR is a sensitive method for a direct diagnosis of a BLV infection (12). The majority of the PCR assays are based on a single assay. However, it has been reported that less than eight genome copies of the provirus could be detected in the background of 2 million negative lymphocytes using nested PCR (13). Therefore, this study used primers designed for 9 segments of different sizes, based on the envelope gene (GenBank AY078387) of the BLV, and a primer set consisting of both the BLV-2 and BLV-8 was used for the nested PCR. The BLV envelope gene was confirmed by sequencing the PCR product, with a 99% confirmation. The confirmation of an infection by the BLV in human leukemic cells and lung cancer cells was carried out by PCR using the BLV-8 primer combination. All 517 cases of human leukemia and 162 cases of lung cancer were negative for the PCR of the BLV proviral DNA indicating that none of these cases had been infected by the BLV.
In conclusion, although meat may have been possibly imported from BLV endemic areas, the BLV infection is not yet a cause of human leukemia or lung cancer in Koreans. These results can be used as a control for further studies on the BLV in Koreans.
Figures and Tables
Fig. 2
PCR amplification of BLV envelope gene.
Target size, 473 bp (BLV-8); M, 1kb plus ladder; Lane 1, BL-3.1; Lane 2, BL-3
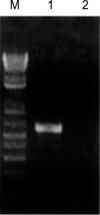
Fig. 3
PCR amplification of BLV envelope gene.
M, 1 kb Plus ladder; 1, BLV-1 (803 bp); 2, BLV-2 (613 bp); 3, BLV-3 (543 bp); 4, BLV-4 (689 bp); 5, BLV-5 (499 bp); 6, BLV-6 (429 bp); 7, BLV-7 (663 bp); 8, BLV-8 (473 bp); 9, BLV-9 (403 bp).
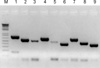
Fig. 4
Determination of PCR sensitivity using nested PCR.
Template, BL-3.1 genomic DNA; A, BLV-3 Primer Set (543 bp); B, BLV-8 Primer Set (473 bp); C, Nested PCR; M, 1 kb Plus Ladder (GIBCO); N, Negative Control; 1, 108 Copies; 2, 107 Copies; 3, 106 Copies; 4, 105 Copies; 5, 104 Copies; 6, 103 Copies; 7, 102 Copies; 8, 10 copies; 9, 1 Copy; 10, 0.1 Copy.
References
1. Johnson ES, Griswold CM. Oncogenic retroviruses of cattle, chickens and turkeys:potential infectivity and oncogenicity for humans. Med Hypotheses. 1996. 46:354–356.
2. Johnson ES, Nicholson LG, Durack DT. Detection of antibodies to avian leucosis/sarcoma viruses (ALSV) and reticuloendotheliosis viruses (REV) in humans by ELISA. Cancer Detect Prev. 1995. 19:394–404.
3. VanLeeuwen JA, Keefe GP, Tremblay R, Power C, Wichtel JJ. Seroprevalence of infection with Mycobacterium avium subspecies paratuberculosis, bovine leukemia virus, and bovine viral diarrhea virus in maritime Canada dairy cattle. Can Vet J. 2001. 42:193–198.
4. Johnson ES, Fischman HR, Matanoski GM, Diamond E. Occurrence of cancer in women in the meat industry. Br J Ind Med. 1986. 43:597–604.

5. Johnson ES, Dalmas D, Noss J, Matanoski GM. Cancer mortality among workers in abattoirs and meatpacking plants: an update. Am J Ind Med. 1995. 27:389–403.

6. Rola M, Kuzmak J. The detection of bovine leukemia virus proviral DNA by PCR-ELISA. J Virol Methods. 2002. 99:33–40.

7. Ghysdael J, Bruck C, Kettmann R, Burny A. Bovine leukemia virus. Curr Top Microbiol Immunol. 1984. 112:1–19.

8. Johnson ES, Nicholson LG, Durack DT. Poultry oncogenic retroviruses and humans. Cancer Detect Prev. 1994. 18:9–31.
9. McClure HM, Keeling ME, Custer RP, Marshak RR, Abt DA, Ferrer JF. Erythroleukemia in two infant chimpanzees fed milk from cows naturally infected with the bovine C-type virus. Cancer Res. 1974. 34:2745–2757.
10. Pearce NE, Smith AH, Howard JK, Sheppard RA, Giles HJ, Teague CA. Non-Hodgkin's lymphoma and exposure to phenoxyherbicides, chlorophenols, fencing work, and meat works employment: a case-control study. Br J Ind Med. 1996. 43:75–83.

11. Johnson ES. Cancer mortality among workers in the meat department of supermarkets. Occup Environ Med. 1991. 51:541–547.

12. Sherman MP, Ehrlich GD, Ferrer JF, Sninsky JJ, Zandomeni R, Dock NL, Poiesz B. Amplification and analysis of specific DNA and RNA sequence of bovine leukemia virus from infected cows by polymerase chain reaction. J Clin Microbiol. 1992. 30:185–191.
13. Ballagi-Pordany A, Klintevall K, Merza M, Klingeborn B, Belak S. Direct detection of bovine leukemia virus infection: practical applicability of a double polymerase chain reaction. Zentralbl Veterinarmed B. 1992. 39:69–77.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download