Abstract
A 47-yr-old man with hepatitis B virus associated liver cirrhosis was admitted to our hospital with diarrhea and generalized edema and diagnosed as protein-losing enteropathy due to intestinal lymphangiectasia by intestinal biopsy and 99mTc albumin scan. During hospitalization, he received subcutaneous octreotide therapy. After 2 weeks of octreotide therapy, follow-up albumin scan showed no albumin leakage, and the serum albumin level was sustained. We speculate that liver cirrhosis can be a cause of intestinal lymphangiectasia and administration of octreotide should be considered for patients with intestinal lymphangiectasia whose clinical and biochemical abnormalities do not respond to a low-fat diet.
Intestinal lymphangiectasia is a relatively rare disorder characterized by dilated intestinal submucosal lymphatics with a loss of lymph into the bowel lumen (1). It has a clinical importance in that hypoproteinemia, edema, lymphopenia, and hypogammagloblinemia can be frequently associated. It is classified into two groups according to its causes; primary type (idiopathic), which has no particular causes, and secondary type, which has underlying causes (2). In Korea, only two cases of intestinal lymphangiectasia have been reported in Korean literatures (3, 4). So far, to our knowledge, there are no reports of intestinal lymphangiectasia associated with cirrhotic ascites.
Until now, there is no specific treatment of proteining-losing enteropathy from intestinal lymphangiectasia. However, Bac et al. (5) reported that octreotide was effective in one patient with the condition. Here we report a case of protein-losing enteropathy induced by intestinal lymphangiectasia in liver cirrhosis patient who was successfully treated with octreotide with a review of the literature.
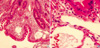
A 47-yr-old male patient was admitted to our hospital with complaints of abdominal pain and diarrhea. Nine years before the admission he had been diagnosed as chronic hepatitis B virus infection, and eight months before admission, he had been diagnosed as liver cirrhosis, but he received no specific treatment. On admission, he complained of diffuse abdominal cramping pain and watery diarrhea. His blood pressure was 100/50 mmHg; heart rate 72 beats/min; respiratory rate 24 breaths/min; and body temperature 38.4℃. He showed an acutely-ill appearance. Physical examination showed mild abdominal distention, diffuse abdominal tenderness, but no rebound tenderness. Laboratory findings were as follows: WBC 2,700/µL (lymphocyte 810/µL), Hb 9.1 g/dL, platelet 53,000/µL, ESR 24 mm/hr, total protein 3.9 g/dL, albumin 2.3 g/dL, total cholesterol 94 mg/dL, ALT/AST 16/57 IU/dL, total bilirubin 1.2 mg/dL, creatinine 1.1 mg/dL, prothrombin time 65%, HBsAg positive, HBsAb negative, HBeAg negative, HBeAb positive, and normal urine analysis. Serum immunoglobulin levels included a normal IgA level of 246 mg/dL (82-453 mg/dL), a normal IgM level of 107 mg/dL (46-304 mg/dL), and a low IgG level of 537 mg/dL (751-1,560 mg/dL). Ascites showed yellowish color and analysis showed WBC 1,300/µL (neutrophil 75%), albumin 0.2 g/dL, triglyceride 8 mg/dL and LDH 36 U/L. Chest radiography finding showed right pleural effusion. Simple abdomen showed mild paralytic ileus. Abdominal ultrasonography showed moderate splenomegaly, ascites, and liver cirrhosis. Cardiovascular assessment showed no signs of pericarditis or valvular abnormalities. Therefore, we diagnosed the patient as having a spontaneous bacterial peritonitis associated with liver cirrhosis and started antibiotics therapy, diuretics, and albumin supply. Notwithstanding daily continuous albumin infusion, hypoalbuminemia was sustained. So we suspected that another causes of hypoalbuminemia might be present and started relevant investigation.With a suspicion of protein-losing enteropathy, albumin scan was performed. It showed protein-losing at jejunum after 2 hr of injection and subsequently moved distal jejunum, ileum, and colon (Fig. 1). In addition, α1 anti-trypsin clearance was increased at 50.03 mL/day (nomal <30 mL/day). Small bowel series and colonocopy showed a nonspecific finding. Then we performed duodenoscopy for the inspection of the suspected lesion on albumin scan. Duodenoscopy showed serosanguinous exudates in the duodenal mucosa, mucosal erosion, snow flake appearances, and erythema in the duodenal third portion (Fig. 2). Histological examination of a biopsy specimen from the duodenum showed markedly dilated lymphatics in the lamina propria (Fig. 3). Based on the microscopic findings, we could make a diagnosis of protein-losing enteropathy induced by intestinal lymphangiectasia.
Since clinical and chemical abnormalities did not respond to low-fat diet, on the 24th day of hospitalization, we started subcutaneous octreotide therapy on the patient (0.1 mg three times daily). After two weeks of octreotide therapy, follow-up albumin scan showed no albumin leakage for three hours after injection. After octreotide therapy, the serum albumin level increased from 2.2 g/dL to 2.7 g/dL after 50 g albumin infusion. It was consistent with the albumin scan finding. During octreotide therapy, the serum albumin level was maintained at 2.8 g/dL without albumin infusion. During follow-up, we stopped octreotide therapy for economic problem of the patient. After one month, the serum albumin level decreased to 1.9 g/dL with continuous albumin infusion and the patient's symptoms recurred with exacerbation of laboratory findings. So the octreotide therapy was resumed. After initiation of octreotide therapy, the serum albumin level increased with time. Until now his serum albumin level has maintained, and there have been no apparent adverse effects from octreotide treatment.
Intestinal lymphangiectasia is characterized by obstruction of the intestinal lymphatics, and the increased lymphatic pressure can cause protein-losing enteropathy and malabsorption. Intestinal lymphangiectasia is a primary disorder in cases of malformation of lymphatic vessels at the intestinal level or in other areas of the body. It can also occur secondary to diseases that may cause intestinal lymphatic obstruction, for example, abdominal or retroperitoneal fibrosis (6). The most common causes of secondary intestinal lymphangiectasia are cardiac diseases and chemotherapeutic, infectious, or toxic substances that are associated with inflammatory processes that may cause retroperitoneal lymph node enlargement. Therefore, all of above condition should be included as a differential diagnosis in intestinal lymphangiectasia. None of these conditions were present in our patient. However, CT or lymphangiogram may be needed in our case to exclude other possible conditions. Intestinal lymphangiectasia primarily affects children and young adults. It usually presents with features of a protein-losing enteropathy; diarrhea, edema, and hypoalbuminemia. Chyluria, chylothorax, chylous ascites, lymphocytopenia and hypocalcemia have also been recognized (7, 11). Endoscopic findings of intestinal lymphangiectasia include a scattered snowflake-like lesion in the mucosa (8). Our case showed a similar snowflake appearance on endoscopy. Consumption of a high-fat meal during the evening before endoscopic evaluation may make these findings more apparent. The diagnosis of intestinal lymphangiectasia is generally established by a biopsy of the intestinal mucosa, which demonstrates dilated mucosal and submucosal lymphatic channels. Because the lesions are frequently patchy, multiple biopsies may be necessary to demonstrate the lymphatic lesion. A low-fat diet with medium-chain triglycerides has been recommended as the initial management of intestinal lymphagiectasia (7). However, the patient's compliance to low-fat diets is not always good, and refractory cases are occasionally observed with low-fat diet. So several intervention strategies have been reported to date, such as corticosteroid, antiplasmin therapy, cytotoxic drug, and even surgery, but the results have not been satisfactory (5, 9). And there are some reports that protein-losing enteropathy due to portal hypertension may be improved by placement of a transjugular intrahepatic portosystemic shunt (11, 13).
Octreotide is a long-acting somatostatin analog that has been shown to suppress gastrointestinal motility and hormone secretion in the pituitary gland, pancreas, and intestine. It also reduces the splanchnic blood flow, decreases endogenous fluid secretion in the jejunum, reduces lymph fluid excretion, and inhibits the absorption of triglycerides (10). There have been only three patients who were treated with octreotide for protein-losing enteropathy from intestinal lymphangiectasia (2, 5, 7). The mechanism of action of octreotide in intestinal lymphangiectasia involves reduction of intestinal blood flow, inhibition of triglyceride absorption, and reduction of lymph flow. The true impact of somatostatin and octreotide on portal hemodynamics remains controversial, and variable changes in portal pressure and intravariceal pressure in response to these drugs have been reported in different studies (14, 15). So, in our case such as intestinal lymphagiectasia combined with portal hypertension, octreotide may effect on portal pressure and therefore improve protein losing. The clinical characteristics in these cases are summarized in Table 1. All of three cases including our case show the efficacy of octreotide in intestinal lymphangiectasia, however, long-term administration of octreotide seems to be required. No adverse effects have been documented in octreotide treatment.
To our knowledge there have been no previous reports of intestinal lymphangiectasia associated with cirrhotic ascites. We think that the lesion in our patient had been acquired from impaired lymph flow, considering the late onset of disease and the fact that the lymphangiectasia appeared to be associated with liver cirrhosis, a disease known to alter lymph flow. Thus we think that intestinal lymphangiectasia should be suspected as a differential diagnosis in refractory hypoalbuminemia in liver cirrhosis patients.
There are several important aspects in our case. First, it is the first case of intestinal lymphangiectasia developing in liver cirrhosis patient. We speculate that liver cirrhosis can be a cause of intestinal lymphangiectasia though the previous mentioned mechanism. Second, through it is a relatively rare disease, we should consider intestinal lymphangiectasia as a differential diagnosis in a patient with protein-losing enteropathy. Third, when we experience continuous hypoalbuminemia despite albumin infusion in a liver cirrhosis patient, we should suspect that presence of another cause of hypo-albuminemia.
Although there can be an economic problem and side effects of long-term therapy of octreotide, the administration of octreotide should be considered for patients with intestinal lymphangiectasia whose clinical and biochemical abnormalities do not respond to a low-fat diet. The mechanism of action is not entirely clear but appears to involve reduction in gut protein loss. Further investigation on the mechanism of action of octreotide is needed. And more fundamental strategies should be introduced in the management of intestinal lymphangiectasia.
Figures and Tables
Fig. 1
Albumin scan shows protein-losing at the jejunum level after 2 hr of injection and subsequently moved distal jejunum, ileum, and colon.

Fig. 2
Duodenoscopy shows serosanguinous exudates in the duodenal mucosa, mucosal erosion, and erythema in the duodenal 3rd portion.

References
1. Sleisenger MH, Fordtran JS. Gastrointestinal disease: pathophysiology, diagnosis, management. 1998. 6th ed. Philadelphia: WB Saunders.
2. Kuroiwa G, Takayama T, Sato Y, Takahashi Y, Fujita T, Nobuoka A, Kukitsu T, Kato J, Sakamaki S, Niitsu Y. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001. 36:129–132.

3. Lee SH, Chang YW, Bak SK, Han JY, Kim JH, Jung YH, Lee BW, Han YS, Dong SH, Kim HJ, Kim BH, Lee JI, Chang R. A case of duodenal lymphangiectasia. Korean J Gastroenterol. 2003. 41:59–63.
4. Park GS, Kwak JY, Kim JS, Kwon TC, Jo YJ. A case of primary Intestinal lymphangiectasia. Korean J Gastrointest Endosc. 1999. 19:634–642.
5. Bac DJ, Van Hagen PM, Postema PT, ten Bokum AM, Zondervan PE, van Blankenstein M. Octreotide for protein-losing enteropathy with intestinal lymphangiectasia. Lancet. 1995. 345:1639.

6. Salvia G, Cascioli CF, Ciccimarra F, Terrin G, Cucchiara S. A case of protein-losing enteropathy caused by intestinal lymphangiectasia in a preterm infant. Pediatrics. 2001. 107:416–417.

7. Ballinger AB, Farthing MJ. Octreotide in the treatment of intestinal lymphangiectasia. Eur J Gastroenterol Hepatol. 1998. 10:699–702.
8. Patel AS, DeRidder PH. Endoscopic appearance and significance of functional lymphangiectasia of the duodenal mucosa. Gastrointest Endosc. 1990. 36:376–378.

9. Mine K, Matsubayashi S, Nakai Y, Nakagawa T. Intestinal lymphangiectasia markedly improved with antiplasmin therapy. Gastroenterology. 1989. 96:1596–1599.

10. Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996. 334:246–254.

11. Heresbach D, Raoul JL, Genetet N, Noret P, Siproudhis L, Ramee MP, Bretagne JF, Gosselin M. Immunological study in primary intestinal lymphangiectasia. Digestion. 1994. 55:59–64.

12. Dousset B, Legmann P, Soubrane O, Chaussade S, Couturier D, Houssin D, Calmus Y. Protein-losing enteropathy secondary to hepatic venous outflow obstruction after liver transplantation. J Hepatol. 1997. 27:206–210.

13. Stanley AJ, Gilmour HM, Ghosh S, Ferguson A, McGilchrist AJ. Transjugular intrahepatic portosystemic shunt as a treatment for protein-losing enteropathy caused by portal hypertension. Gastroenterology. 1996. 111:1679–1682.

14. Garcia-Pagan JC, Escorsell A, Moitinho E, Bosch J. Influence of pharmacological agents on portal hemodynamics: basis for its use in the treatment of portal hypertension. Seminar Liver Dis. 1999. 19:427–438.
15. D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Seminar Liver Dis. 1999. 19:475–505.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download