Abstract
Background
Hematology analyzers may ineffectively recognize abnormal cells, and manual differential counts may be imprecise for leukopenic samples. We evaluated the efficacy of the Hematoflow method for determining the leukocyte differential in leukopenic samples and compared this method with the manual differential method.
Methods
We selected 249 blood samples from 167 patients with leukopenia (WBC counts, 500-2,000/µL) for analysis in this study. The EDTA-anticoagulated blood samples were analyzed using an automatic blood cell counter (DxH800; Beckman Coulter, USA) and flow cytometry (FC 500; Beckman Coulter) by using Cytodiff reagent and analysis software (Beckman Coulter). Hematoflow results were selected or calculated from DxH800 and Cytodiff results. Two trained pathologists performed a manual differential count by counting 50-100 cells.
Results
The precision of the Hematoflow method was superior to that of the manual method in counting 5 leukocyte subpopulations, immature granulocytes (IGs), and blasts. Blasts were detected in all 45 cases (100%) by Hematoflow. The correlation of the Cytodiff blast count to the reference count was high (r = 0.8325). For all other cell populations, the correlation of the Hematoflow results with the reference count was stronger than that of the other manual counts with the reference count.
Conclusions
The Hematoflow differential counting method is more reproducible and sensitive than manual counting, and is relatively easy to perform. In particular, this method detected leukemic blasts more sensitively than manual differential counts. The Hematoflow method is a very useful supplement to automated cell counting.
The majority of hematology cell analyzers are useful for quantitative enumeration of leukocytes, but they are relatively ineffective in qualitative recognition of abnormal cells [1]. Hematology cell analyzers can detect abnormal differential patterns or abnormal cells and "flag" them. However, in most cases, the presence of abnormal cells must be established by microscopic examination of blood smears [2, 3]. Manual differential counting is the reference method for white blood cell (WBC) differential counts, but it is labor-intensive and time-consuming with variable reproducibility and cannot distinguish between cells on the basis of qualitative differences [4]. The incidence of leukopenia has markedly increased in hospital laboratories in recent years, largely due to an increase in the number of cancer and leukemia patients receiving chemotherapy or radiotherapy [5, 6]. Manual differential counts of leukopenic samples are notoriously imprecise, which can be attributed to the insufficient number of countable cells in these samples. Further, the classification of cells in these samples is generally both difficult and time-intensive [7]. Rapid and accurate confirmation of abnormal cells by flow cytometry is required to reach a final diagnostic conclusion.
One theoretical advantage of flow cytometric assessment of cells is the identification of cell types that cannot be identified by the current morphologic differentiation techniques [8-10]. Recently, the Hematoflow method was introduced (Beckman Coulter, Miami, FL, USA). This method combines an automatic hematology cell analyzer (DxH800; Beckman Coulter), flow cytometry (FC500; Beckman Coulter), and Cytodiff (5-colors 6-antibodies flow cytometry using premixed reagent & analysis software; Beckman Coulter) [11]. In this study, we evaluated the efficacy of determining the leukocyte differential by using the Hematoflow method in leukopenic samples and compared it with the manual differential method.
Two hundred forty-nine EDTA-anticoagulated blood samples from 167 patients (88 males and 79 females; age, 1-75 yr; mean age, 38 yr) who had a WBC count of 500-2,000/µL in routine CBC were selected for analysis. The patients' diagnoses were as follows: 65 cases of AML, 34 cases of ALL, 15 cases of aplastic anemia, 14 cases of malignant lymphomas, 10 cases of solid tumors, 10 cases of MDS, 1 case of paroxysmal nocturnal hemoglobinuria (PNH), and 18 cases of other hematologic diseases. Blasts were discovered during the reference count in 36 of 125 AML samples and 9 of 46 ALL samples. None of the samples from MDS patients harbored blasts. This study was approved by the Institutional Review Board of the Catholic University of Korea College of Medicine.
Blood samples were analyzed with an automatic blood cell analyzer (DxH800) for routine CBC, and 8,920 leukocytes were counted per sample. We then analyzed the same blood samples for determining the leukocyte differential using flow cytometry (FC500) in conjunction with a premixed Cytodiff reagent and analysis software. The Cytodiff panel included CD36-FITC, CD2-PE, CD294-PE, CD19-ECD, CD16-PC5, and CD45-PC7 antibodies. The leukocytes were differentiated into 16 cell populations (B-lymphocytes, CD16- T-lymphocytes, CD16+ T and NK cells, T and NK lymphocytes, total lymphocytes, CD16 monocytes, CD16+ monocytes, total monocytes, immature granulocytes [IGs], total eosinophils, mature neutrophils, total neutrophils, B blasts, T blasts, non-B-non-T blasts, and total basophils; Fig. 1). Analysis procedures were conducted in accordance with the manufacturer's manual. In brief, 100 µL of whole blood samples was mixed with 10 µL of Cytodiff reagent and incubated for 20 min at room temperature. Red blood cells were broken down with lysing solution (Versalyse solution; Beckman Coulter) for 15 min. Without washing, 10,000 cells were collected using a flow cytometer (FC500) and the Cytodiff results were analyzed automatically by the analysis software. The analysis software is self-gating and separates populations by automatic logic pathways. As instructed by the manual, the gates were only adjusted in cases of large debris contamination or incomplete separation of basophils and myeloblasts. All samples were analyzed in duplicate. All the Hematoflow results, except eosinophil and IG counts, were obtained using the Cytodiff reagent. We used the DxH800 eosinophil count as the Hematoflow eosinophil count because the DxH800 eosinophil count was more highly correlated to the reference eosinophil count than that obtained using Cytodiff. Hematoflow IG count was calculated by subtracting the DxH800 eosinophil count from the sum of the Cytodiff IG count and Cytodiff eosinophil count, because the IGs and eosinophils were not well separated in Cytodiff counting.
A trained hematology technician and a hematopathologist did manual differential counts by counting 50-100 cells. We selected the manual counts done by hematopathologist as reference counts. Manual counting may increase the sampling error rate [7]. We added the number of metamyelocytes, myelocytes, and promyelocytes to obtain the number of IGs in the manual count.
The average acquisition time for 20 leukopenic samples (10,000 events) by Hematoflow was approximately 90 min, including incubation and reading time, and the active labor time was 15 min. In Cytodiff counting, gates were adjusted in 19 out of 247 cases (7.7%). In 2 cases, adjustments were made because of large debris contamination and in 17 cases, they were made because of incomplete separation between basophils and myeloblasts. In both cases of large debris contamination, the contamination was only observed during the first run, but not during the second run.
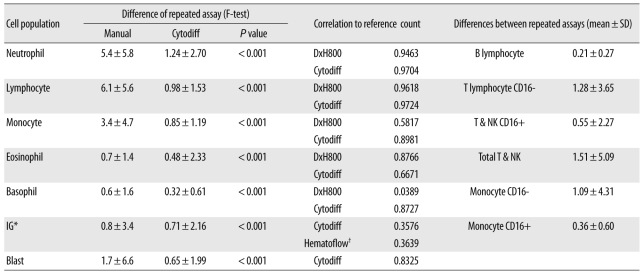
The repeatability of the Hematoflow method in counting 5 leukocyte subpopulations, IGs, and blasts was superior to that of the manual differential method (Table 1). The mean differences between repeated Cytodiff counts were 1.24±2.70%, 0.98±1.53%, and 0.85±1.19% for neutrophils, lymphocytes and monocytes, respectively. When counted manually, the mean differences between repeated counts were much higher (5.4±5.8%, 6.1±5.6%, and 3.4±4.7%, respectively, P<0.001). Cytodiff counts for neutrophils, lymphocytes, and monocytes were highly correlated with the reference counts (r=0.9704, r=0.9724, and r=0.8981, respectively).
The mean difference between repeated eosinophil assays by the Cytodiff count was 0.48±2.33% and was lower than that in the case of manual counting (0.7±1.4%, P<0.001). However, in the case of the eosinophil counts, the correlation of the reference count with the DxH800 count (r=0. 8766) was higher than that with the manual count (r=0. 8222) or the Cytodiff count (r=0.6671). Therefore, eosinophil counting by DxH800 was used as the eosinophil count in Hematoflow.
The distinction between basophil and non-B-non-T blast populations was not particularly clear. In such cases, the cell populations were considered to represent either basophils or non-B-non-T blasts on the basis of the median fluorescence level (<3.5: non-B-non-T blasts, ≥3.5: basophils). The mean difference between repeated basophil assays by Cytodiff count was 0.32±0.61% and was slightly lower than that in the case of manual counting (0.6±1.6%, P<0.001). The correlation of the basophil count to the reference count was high in the case of Cytodiff count (r=0.8727) and was higher than that for manual counting (r=0.7886). DxH800 basophil counts, however, were poorly correlated with the reference count (r=0.0389; Table 1). When non-B-non-T blasts were not present, we obtained clear basophil signals in Cytodiff (Fig. 2).
Blasts were detected in all 45 cases (100%) by Hematoflow method and in 17 cases (37.8%) by manual counting method (Figs. 2, 3). The mean difference between repeated blast assays by Hematoflow count was 0.65±1.99% and was lower than that obtained by manual counts (1.7±6.6%, P<0.001). The correlation of the blast count to the reference count was similar for the Cytodiff count (r=0.8325) and the manual count (r=0.8627).
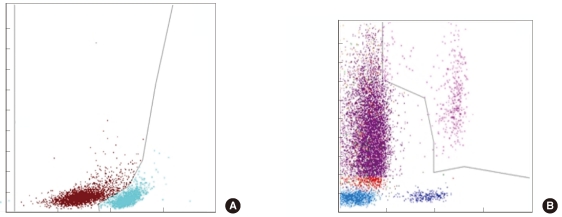
The Hematoflow IG count correlated more strongly with the reference count than the Cytodiff IG count (Table 1). The mean difference between repeated IG assays by Hematoflow count was 0.71±2.16% and was slightly lower than that in the case of the manual count (0.8±3.4%, P<0.001). The correlation of the Hematoflow IG count to the reference count (r=0.3639) was higher than that of the manual count (r=0.2170). Type III granulocytes were sometimes counted as IGs, especially in 1 PNH case, because the type III neutrophils are CD16 negative cells (Fig. 4).
The slides from leukopenic samples often do not have a sufficient number of cells to count, and it is difficult to observe a cell distribution in the ideal zone. The majority of leukopenic samples are collected from patients receiving chemotherapy and/or radiotherapy, which can cause altered morphologies of the cells [12]. This makes the manual differential count of leukopenic samples more difficult. However, the absolute neutrophil count (ANC) or presence of blasts is critically important for patients. The average time required for the Hematoflow counts of 20 leukopenic samples (10,000 events) was approximately 90 min, including incubation and reading time, and the active labor time was only 15 min (less than 1 min per case). Manual differential counting requires slide making, staining, drying, and microscopic examination. It is very time-consuming and it is difficult to conduct differential counts of leukopenic samples. The throughput of manual differential counting of leukopenic samples depends on the leukocyte count, skill of the personnel, and active labor time, but it is usually more than 5 min per sample. Therefore, the throughput of the Hematoflow method is acceptable.
Gates were adjusted in 19 of 247 Cytodiff cases (7.7%) due to large debris contamination (2 cases) and incomplete separation between basophils and myeloblasts (17 cases). The 2 cases involving large debris contamination exhibited this problem only in the first run, but not in a repeated run. Therefore, repeated analysis is recommended when large debris is present. As large debris is rarely present in samples from non-leukemia patients, these samples do not require any additional work. Therefore, this method may prove more valuable for non-leukemic patients than for leukemic patients.
The repeatability of the Hematoflow method was superior to that of the manual differential counting method in the counting of 5 leukocyte subpopulations, IGs, and blasts. Additionally, the correlation of counts obtained using the Hematoflow method to the reference counts was also quite good. This means that in leukopenic samples, the leukocyte differential obtained using the Hematoflow method is more reproducible and sensitive than that obtained using other methods, including the manual differential method. In particular, the mean difference between repeated Cytodiff neutrophil assays was far lower than that between repeated manual counts. This might facilitate reproducible ANC counts in patients with leukopenia.
The mean difference between repeated monocyte assays by Cytodiff count was much lower than that by manual counts. The reference monocyte count showed high correlation with the Cytodiff count (r=0.8981), which was much higher than that with the DxH800 count (r=0.5817). This means that the morphology of other leukocytes in the leukopenic samples could have been seriously altered, possibly by processes such as vacuolation, making it difficult to visually differentiate them from monocytes.
The eosinophil count from DxH800 was superior to that of the other methods tested. Hematology cell analyzers are quite effective in eosinophil counting [13, 14]. The separation of IGs and eosinophils by automatic gating in the Cytodiff method is not an efficient method for distinguishing IGs from eosinophils. Therefore, we recommend using the DxH800 eosinophil results and the calculated IG count as the Hematoflow values for these cell types.
While other researchers have reported a low correlation between the Cytodiff basophil count and reference counts [8], we found a high correlation between these counts (r=0.8727). This is due to CD2 + CRTH2. The CRTH2 is expressed in activated T-cells, eosinophils, and basophils. Separation between basophils and non-B-non-T blast populations was not effective in the Hematoflow method. In these cases, the cell populations were counted as either basophils or non-B-non-T blasts using the median fluorescence level (<3.5: non-B-non-T blasts, ≥3.5: basophils). Because clear basophil signals were obtained in cases without non-B-non-T blasts, this calculation was not required for non-AML samples. The blast-detection rate using Hematoflow was 100%, which is much higher than the rate achievable by microscopic examination. The detection of rare blasts in leukemia patients is very important [15]. The precision of blast detection by Hematoflow method was superior to that of the manual counting method. The correlation of the blast count by the Hematoflow method to the reference count was similar. It may be useful to screen samples for the presence of blasts using Cytodiff. If blasts are detected by Cytodiff, confirmation of the blasts by slide review is recommended, especially in new patients.
The repeatability of remaining cell population counts was excellent when the Hematoflow method was used, even for counts made from leukopenic samples. The significance of differential counts of subsets of lymphocytes has been well established, and each cell population has a distinct clinical significance [16-18].
Cytodiff categorizes the PNH type III granulocytes as IGs, because the type III neutrophils are CD16 negative [19]. If a higher than normal Hematoflow IG count is detected in a patient without a reason for the left shift of neutrophils, PNH should be suspected.
In this study, we evaluated a new WBC differential method. The Hematoflow method uses flow cytometry (FC500) together with a hematology cell analyzer (DxH800). The Hematoflow differential counting method is a more reproducible and sensitive method than manual counting, and is relatively easy to perform. In particular, the Hematoflow method is much more sensitive than the manual differential counting method in the detection of leukemic blasts. The Hematoflow method provides more cell population data [11] than the manual differential count, including blast lineages. In conclusion, the Hematoflow method is very useful in supplementing the automated cell counting. The technique is expected to be even more useful if data from the hematology cell analyzer and flow cytometry can be automatically combined, and any required calculations become automated.
References
1. Guerti K, Vertessen F, Daniëls L, Van Der Planken M. Performance evaluation of the PENTRA 60C+ automated hematology analyzer and comparison with the ADVIA 2120. Int J Lab Hematol. 2009; 31:132–141. PMID: 19267810.
2. Novis DA, Walsh M, Wilkinson D, St Louis M, Ben-Ezra J. Laboratory productivity and the rate of manual peripheral blood smear review: a college of American pathologists Q-probes study of 95,141 complete blood count determinations performed in 263 institutions. Arch Pathol Lab Med. 2006; 130:596–601. PMID: 16683868.

3. Barnes PW, McFadden SL, Machin SJ, Simson E. The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 2005; 11:83–90. PMID: 16024331.

4. Fuentes-Arderiu X, García-Panyella M, Dot-Bach D. Between-examiner reproducibility in manual differential leukocyte counting. Accred Qual Assur. 2007; 12:653–645.

5. Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer. 2010; 18:529–541. PMID: 20191292.

6. Mac Manus M, Lamborn K, Khan W, Varghese A, Graef L, Knox S. Radiotherapy-associated neutropenia and thrombocytopenia: analysis of risk factors and development of a predictive model. Blood. 1997; 89:2303–2310. PMID: 9116273.

7. Pierre RV. The demise of the eyecount leukocyte differential. Clin Lab Med. 2002; 22:279–297. PMID: 11933579.
8. Cherian S, Levin G, Lo WY, Mauck M, Kuhn D, Lee C, et al. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytometry B Clin Cytom. 2010; 78:319–328. PMID: 20533390.

9. Björnsson S, Wahlström S, Norström E, Bernevi I, O'Neill U, Johansson E, et al. Total nucleated cell differential for blood and bone marrow using a single tube in a five-color flow cytometer. Cytometry B Clin Cytom. 2008; 74:91–103. PMID: 18061952.

10. Roussel M, Benard C, Ly-Sunnaram B, Fest T. Refining the white blood cell differential: the first flow cytometry routine application. Cytometry A. 2010; 77:552–563. PMID: 20506466.

11. Faucher JL, Lacronique-Gazaille C, Frébet E, Trimoreau F, Donnard M, Bordessoule D, et al. "6 Markers/5 Colors" Extended White Blood Cell Differential by Flow Cytometry. Cytometry A. 2007; 71:934–944. PMID: 17879238.

12. Shafer JA. Blood and marrow morphology in acute leukemia patients receiving chemotherapy: a photo-essay. Am J Med Technol. 1983; 49:77–90. PMID: 6573133.
13. Jean A, Boutet C, Lenormand B, Callat MP, Buchonnet G, Barbay V, et al. The new haematology analyzer DxH 800: an evaluation of the analytical performances and leucocyte flags, comparison with the LH 755. Int J Lab Hematol. 2010; 8. 16. [Epub ahead of print].

14. Koenn ME, Kirby BA, Cook LL, Hare JL, Hall SH, Barry PM, et al. Comparison of four automated hematology cell analyzers. Clin Lab Sci. 2001; 14:238–242. PMID: 11760821.
15. Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999; 38:139–152. PMID: 10440852.

16. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008; 112:1570–1580. PMID: 18725575.

17. Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006; 211:214–224. PMID: 16824130.
18. Thalhammer-Scherrer R, Veitl M, Exner M, Schneider B, Geissler K, Simonitsch I, et al. Role of immunological lymphocyte subset typing as a screening method for lymphoid malignancies in daily routine practice. Cytometry. 2000; 42:5–10. PMID: 10679737.

19. Richards SJ, Barnett D. The role of flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria in the clinical laboratory. Clin Lab Med. 2007; 27:577–590. PMID: 17658408.

Fig. 1
An example of CytoDiff results. Sixteen cell populations are displayed in different colors with complicated gates.
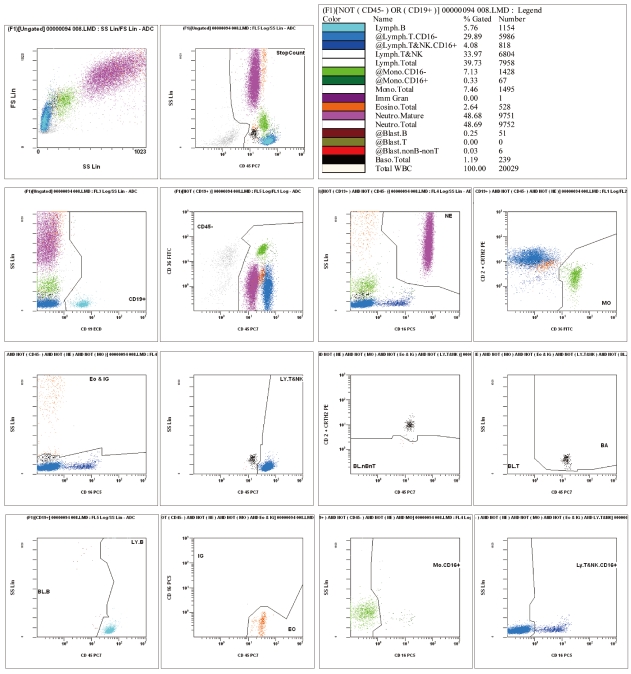
Fig. 2
(A) Non-B-non-T blasts (red) and basophils (black) using the CD2+CD294 expression pattern. (B) The basophils show higher fluorescence than the non-B-non-T blasts.
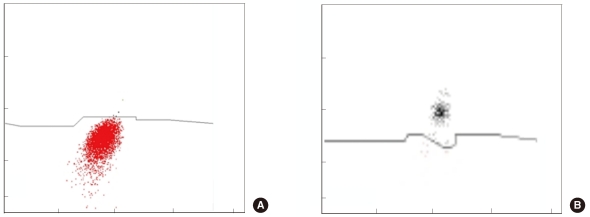
Fig. 3
(A) B lymphoblasts (brown) and B lymphocytes (sky-blue) using the CD19+ cell population are differentiated by CD45 expression level. (B) Because the promyelocytes are included in IGs, acute promyelocytic leukemic cells are counted as IGs (purple).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download