Abstract
Background:
Bloodstream infection (BSI) is associated with a high mortality rate. Since the origin of infection is demonstrated in approximately 2/3rds of cases, early and established biomarkers are warranted. We evaluated the clinical performances of automated procalcitonin (PCT) and C-reactive protein (CRP) assays for the quantitative detection of BSI. Analytical performance of the VIDAS® B · R · A · H · M · S PCT assay (bioMérieux, France) was assessed and also compared with the semiquantitative PCT-Q test (B · R · A · H · M · S Aktiengesellschaft, Germany).
Methods:
We prospectively included consecutive patients divided into 3 groups at the Dong-A University Medical Center. Patients were categorized according to the criteria of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (ACCP/SCCM), and also on the basis of catheter-associated bacteremia.
Results:
A total 77 patients were enrolled. All mean values of PCT and PCT-Q were consistent with the reference value. Measured PCT concentrations showed good linearity (r=0.983). The between-run, within-run, and total imprecisions were below 5%. The PCT levels in gram-negative bacteremia were significantly higher than those in gram-positive bacteremia. Furthermore, the PCT concentrations were significantly different among non-infection, bacteremia, sepsis, severe sepsis, and septic shock groups. Our study showed that PCT >0.3 ng/mL had 95.0% sensitivity and 97.3% specificity, whereas CRP >5.46 mg/dL had 85.0% sensitivity and 86.5% specificity for diagnosing sepsis.
REFERENCES
1.Mylotte JM., Kahler L., McCann C. Community-acquired bacteremia at a teaching versus a nonteaching hospital: impact of acute severity of illness on 30-day mortality. Am J Infect Control. 2001. 29:13–9.

2.Weinstein MP., Towns ML., Quartey SM., Mirrett S., Reimer LG., Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997. 24:584–602.

3.Munson EL., Diekema DJ., Beekmann SE., Chapin KC., Doern GV. Detection and treatment of bloodstream infection: laboratory reporting and antimicrobial management. J Clin Microbiol. 2003. 41:495–7.

4.Khatib R., Saeed S., Sharma M., Riederer K., Fakih MG., Johnson LB. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2006. 25:181–5.

5.Angus DC., Linde-Zwirble WT., Lidicker J., Clermont G., Carcillo J., Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001. 29:1303–10.

6.Sudhoff T., Giagounidis A., Karthaus M. Serum and plasma parameters in clinical evaluation of neutropenic fever. Antibiot Chemother. 2000. 50:10–9.
7.Assicot M., Gendrel D., Carsin H., Raymond J., Guilbaud J., Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993. 341:515–8.

8.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992. 20:864–74.
9.Muller B., Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Med Wkly. 2001. 131:595–602.
10.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach: approved guideline. Document EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.
11.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods: approved guideline. Document EP5-A2. 2nd ed.Wayne, PA: Clinical and Laboratory Standards Institute;2004.
12.Schuetz P., Christ-Crain M., Huber AR., Muller B. Long-term stability of procalcitonin in frozen samples and comparison of Kryptor and VIDAS automated immunoassays. Clin Biochem. 2010. 43:341–4.

13.Jongwutiwes U., Suitharak K., Tiengrim S., Thamlikitkul V. Serum procalcitonin in diagnosis of bacteremia. J Med Assoc Thai. 2009. ;92 (Suppl 2):S. 79–87.
14.Levy MM., Fink MP., Marshall JC., Abraham E., Angus D., Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003. 31:1250–6.

15.Brunkhorst FM., Wegscheider K., Forycki ZF., Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000. 26(Suppl 2):148–52.

16.Al-Nawas B., Krammer I., Shah PM. Procalcitonin in diagnosis of severe infections. Eur J Med Res. 1996. 1:331–3.
17.Simon L., Gauvin F., Amre DK., Saint-Louis P., Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004. 39:206–17.

18.Uzzan B., Cohen R., Nicolas P., Cucherat M., Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006. 34:1996–2003.

19.Nakamura A., Wada H., Ikejiri M., Hatada T., Sakurai H., Matsushima Y, et al. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock. 2009. 31:586–91.

Fig. 1.
Flow diagram of the subjects included in this study.
Abbreviations: B/C, blood culture; ER, emergency room; SIRS, systemic inflammatory response syndrome.
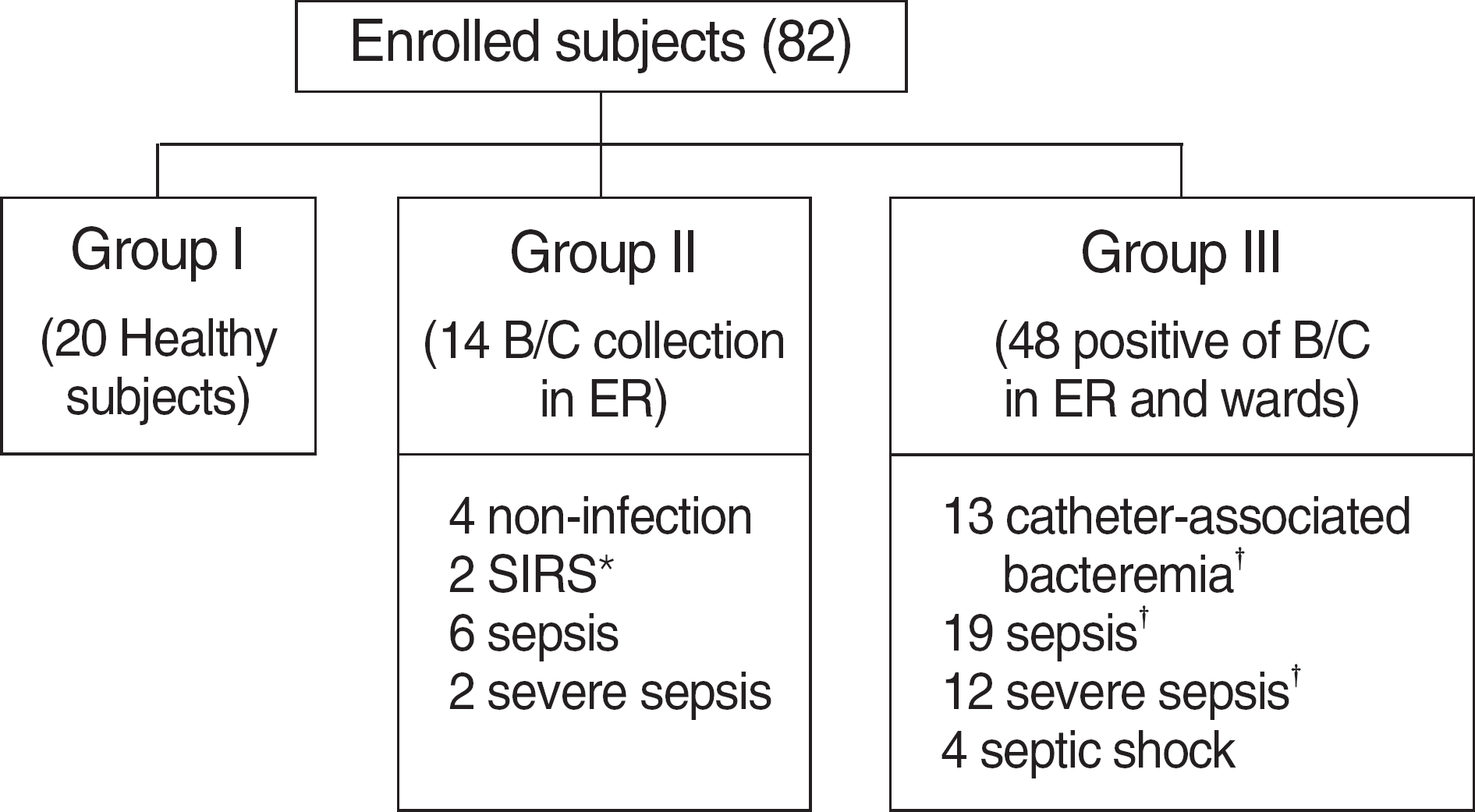
Fig. 3.
Serum PCT level (A) and CRP level (B) in patients with no infection, bacteremia (catheter associated), sepsis, severe sepsis, and septic shock. Data are presented as box plots with median lines, 25- and 75-percentile boxes, and 10- and 90-percentile error bars. A log scale is used for the Y-axis in (A).
Abbreviations: PCT, procalcitonin; CRP, C-reactive protein.
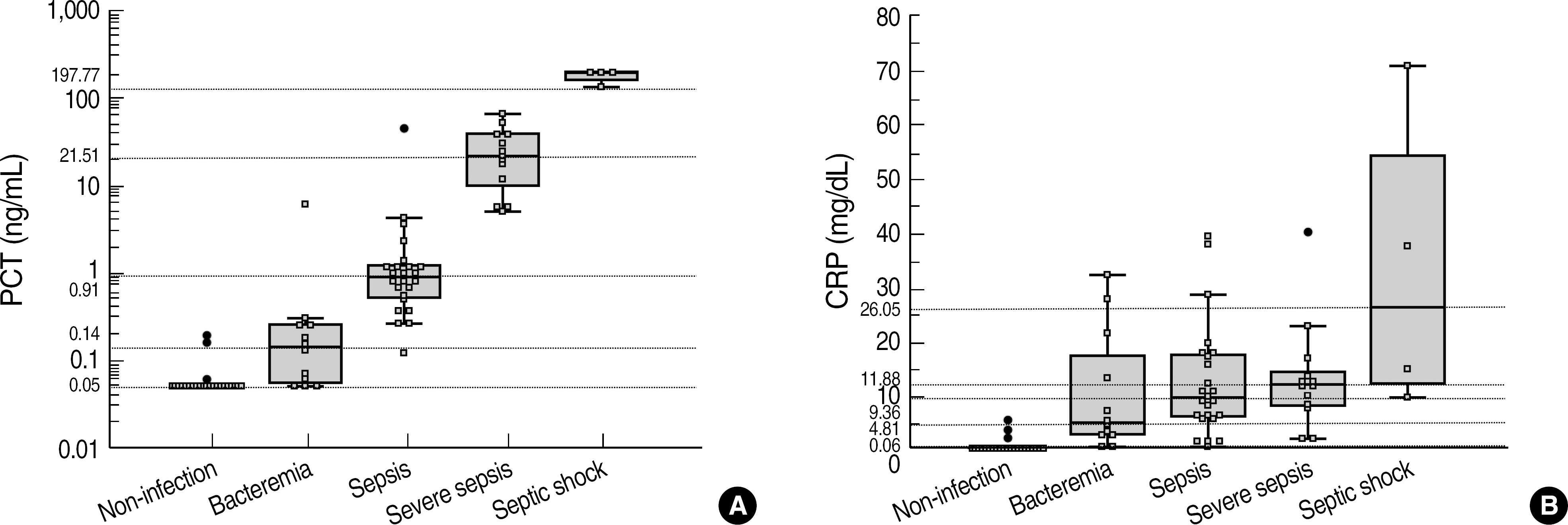
Fig. 4.
The ROC curves of PCT (AUC, 0.982) and CRP (AUC, 0.871) for the diagnosis of sepsis.
Abbreviations: PCT, procalcitonin; CRP, C-reactive protein; AUC, area under the curve.
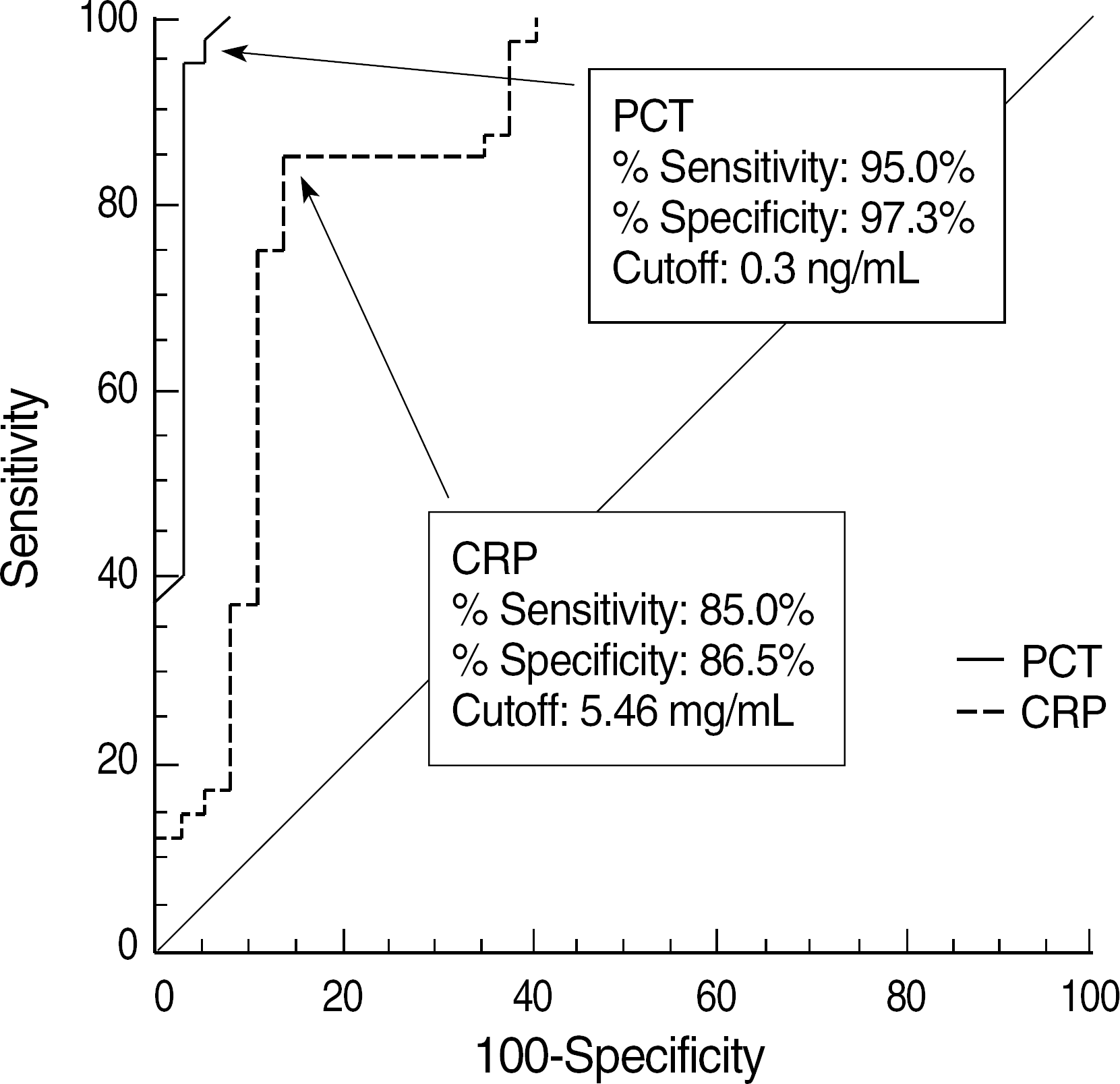
Table 1.
Comparison of procalcitonin measured using VIDAS® PCT and PCT-Q assays in 28 patients in groups I (N=12) and II (N=16)
| PCT-Q (ng/mL) | Total | ||||
|---|---|---|---|---|---|
| <0.5 | 0.5-2 | 2-10 | ≥10 | ||
| Agreement | 15 | 2 | 3 | 2 | 22 |
| Disagreement | 0 | 5∗ | 1† | 0 | 6 |
| Total | 15 | 7 | 4 | 2 | 28 |
Table 2.
Relationship of between PCT and CRP concentrations based on pathogens




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download