Abstract
Background
Acinetobacter baumannii is an aerobic, gram-negative, glucose-nonfermenting bacterium, which has emerged as a serious opportunistic pathogen. In recent years, the increasing instance of carbapenem-resistant A. baumannii producing metallo-β-lactamases (MBLs) or OXA-type β-lactamases is causing a serious clinical problem. In this study, we investigated the prevalence of Ambler class A, B, and D β-lactamases and their extended-spectrum derivatives in carbapenem-resistant A. baumannii isolates.
Methods
A total of 31 consecutive, non-duplicate, carbapenem-resistant A. baumannii were isolated from three university hospitals in the Chungcheong province of Korea. The modified Hodge and inhibitor-potentiated disk diffusion tests were conducted for the screening of carbapenemase and MBL production, respectively. PCR and DNA sequencing were performed for the detection of β-lactamase genes. We also employed the enterobacterial repetitive intergenic consensus (ERIC)-PCR method for the epidemiologic study.
Results
Twenty-three of 31 isolates harbored blaOXA-2 (51.6%), blaOXA-23 (22.6%), blaIMP-1 (48.4%), and blaVIM-2 (3.2%). All of the OXA-2-producing strains also evidenced MBLs. The strains that harbored blaOXA-23 were isolated only in hospital C, and only in a limited fashion. The ERIC-PCR pattern of the five OXA-23 strains indicated that the isolates were closely related in terms of clonality. The six strains producing IMP-1 isolated from hospital A were confirmed to be identical strains.
Conclusions
A. baumannii strains harboring IMP-1 or OXA-type β-lactamases are currently widely distributed throughout the Chungcheong province of Korea. The most notable finding in this study was that a blaOXA-2-producing A. baumannii harboring MBL, which has not been previously reported, can also lead to outbreaks.
REFERENCES
1.Bergogne-bérézin E., Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996. 9:148–65.
2.Poirel L., Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006. 12:826–36.
3.Fernández-Cuenca F., Martínez-Martinez L., Conejo MC., Ayala JA., Perea EJ., Pascual A. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother. 2003. 51:565–74.
4.Clark RB. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33-36 kDa outer membrane protein. J Antimicrob Chemother. 1996. 38:245–51.
5.Afzal-shah M., Woodford N., Livermore DM. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D beta-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2001. 45:583–8.
6.Naas T., Levy M., Hirschauer C., Marchandin H., Nordmann P. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-23 in a tertiary care hospital of Papeete, French Polynesia. J Clin Microbiol. 2005. 43:4826–9.
7.Lauretti L., Riccio ML., Mazzariol A., Cornaglia G., Amicosante G., Fontana R, et al. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999. 43:1584–90.
8.Lee K., Ha GY., Shin BM., Kim JJ., Kang JO., Jang SJ, et al. Metallo-beta-lactamase-producing Gram-negative bacilli in Korean Nationwide Surveillance of Antimicrobial Resistance group hospitals in 2003: continued prevalence of VIM-producing Pseudomonas spp. and increase of IMP-producing Acinetobacter spp. Diagn Microbiol Infect Dis. 2004. 50:51–8.
9.Nishio H., Komatsu M., Shibata N., Shimakawa K., Sueyoshi N., Ura T, et al. Metallo-beta-lactamase-producing gram-negative bacilli: laboratory-based surveillance in cooperation with 13 clinical laboratories in the Kinki region of Japan. J Clin Microbiol. 2004. 42:5256–63.
10.Wachino J., Doi Y., Yamane K., Shibata N., Yagi T., Kubota T, et al. Molecular characterization of a cephamycin-hydrolyzing and inhibitor-resistant class A beta-lactamase, GES-4, possessing a single G170S substitution in the omega-loop. Antimicrob Agents Chemother. 2004. 48:2905–10.
11.Aubert D., Poirel L., Chevalier J., Leotard S., Pages JM., Nordmann P. Oxacillinase-mediated resistance to cefepime and susceptibility to ceftazidime in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2001. 45:1615–20.
12.Lolans K., Rice TW., Munoz-Price LS., Quinn JP. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob Agents Chemother. 2006. 50:2941–5.
13.Scaife W., Young HK., Paton RH., Amyes SG. Transferable imipenem-resistance in Acinetobacter species from a clinical source. J Antimicrob Chemother. 1995. 36:585–6.
14.Girlich D., Naas T., Leelaporn A., Poirel L., Fennewald M., Nordmann P. Nosocomial spread of the integron-located veb-1-like cassette encoding an extended-spectrum beta-lactamase in Pseudomonas aeruginosa in Thailand. Clin Infect Dis. 2002. 34:603–11.
15.Poirel L., Weldhagen GF., Naas T., De Champs C., Dove MG., Nordmann P. GES-2, a class A beta-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob Agents Chemother. 2001. 45:2598–603.
16.Versalovic J., Koeuth T., Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991. 19:6823–31.
17.Watanabe M., Iyobe S., Inoue M., Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991. 35:147–51.
18.Schreckenberger PC., Graevenitz A. Acinetobacter, Achromobacter, Alcaligenes, Moraxella, Methylobacterium, and other nonfermentative gram-negative rods. Murray PR, Baron EJ, editors. Manual of clinical microbiology. 7th ed.Washington D.C.: ASM Press;1999. 539–60.
19.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement, M100-S15. Wayne, PA: CLSI;2005.
20.Jeon BC., Jeong SH., Bae IK., Kwon SB., Lee K., Young D, et al. Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 beta-lactamase in Korea. J Clin Microbiol. 2005. 43:2241–5.
21.Oh EJ., Lee S., Park YJ., Park JJ., Park K., Kim SI, et al. Prevalence of metallo-beta-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii in a Korean university hospital and comparison of screening methods for detecting metallo-beta-lactamse. J Microbiol Methods. 2003. 54:411–8.
22.De Champs C., Poirel L., Bonnet R., Sirot D., Chanal C., Sirot J, et al. Prospective survey of beta-lactamases produced by ceftazidime-resistant Pseudomonas aeruginosa isolated in a French hospital in 2000. Antimicrob Agents Chemother. 2002. 46:3031–4.
23.Heritier C., Dubouix A., Poirel L., Marty N., Nordmann P. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58. J Antimicrob Chemother. 2005. 55:115–8.
24.Kang JH., Bae IK., Kwon SB., Jeong SH., Lee JW., Lee WG, et al. Prevalence of Ambler class A extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates in Korea. Korean J Clin Microbio. 2005. 8:17–25. (강지혜, 배일권, 권수봉, 정석훈, 이종욱, 이위교 등. Ambler class A extended-spectrum -lactamase 생성 Escherichia coli와 Klebsiella pneumoniae의 국내 분리 현황. 대한임상미생물학회지 2005;8: 17-25.).
25.Naas T., Benaoudia F., Massuard S., Nordmann P. Integron-located VEB-1 extended-spectrum beta-lactamase gene in a Proteus mirabilis clinical isolate from Vietnam. J Antimicrob Chemother. 2000. 46:703–11.
26.Park JH., Lee SH., Jeong SH., Kim BN., Kim KB., Yoon JD, et al. Characterization and prevalence of Escherichia coli and Klebsiella pneumoniae isolates producing an extended spectrum β-lactamase from Korean hospitals. Korean J Lab Med. 2003. 23:18–24. (박정호, 이상희, 정석훈, 김빛나, 김경보, 윤종득 등. 전국 주요 병원에서 분리된 Escherichia coli와 Klebsiella pneumoniae의 Extended-Spectrum -lactamase 생성 현황과 특성. 대한진단검사의학회지 2003;23: 18-24.).
27.Jeong SH., Bae IK., Park KO., An YJ., Sohn SG., Jang SJ, et al. Outbreaks of imipenem-resistant Acinetobacter baumannii producing carbapenemases in Korea. J Microbiol. 2006. 44:423–31.
28.Nordmann P., Guibert M. Extended-spectrum β-lactamases in Pseudomonas aeruginosa. J Antimicrob Chemother. 1998. 42:128–31.
29.Lee S., Park YJ., Kim M., Lee HK., Han K., Kang CS, et al. Prevalence of Ambler class A and D beta-lactamases among clinical isolates of Pseudomonas aeruginosa in Korea. J Antimicrob Chemother. 2005. 56:122–7.
30.Tognim MC., Gales AC., Penteado AP., Silbert S., Sader HS. Dissemination of IMP-1 metallo-beta-lactamase-producing Acinetobacter species in a Brazilian teaching hospital. Infect Control Hosp Epidemiol. 2006. 27:742–7.
31.Dalla-Costa LM., Coelho JM., Souza HA., Castro ME., Stier CJ., Bragagnolo KL, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the OXA-23 enzyme in Curitiba, Brazil. J Clin Microbiol. 2003. 41:3403–6.
32.Playford EG., Craig JC., Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007. 65:204–11.
33.Wilks M., Wilson A., Warwick S., Price E., Kennedy D., Ely A, et al. Control of an outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus colonization and infection in an intensive care unit (ICU) without closing the ICU or placing patients in isolation. Infect Control Hosp Epidemiol. 2006. 27:654–8.
Fig. 1.
ERIC-PCR patterns of genomic DNA from seven clinical isolates of A. baumannii harboring blaoxa-23. Lane M1 and M2 are 1 kb and 100 bp DNA size markers, respectively.
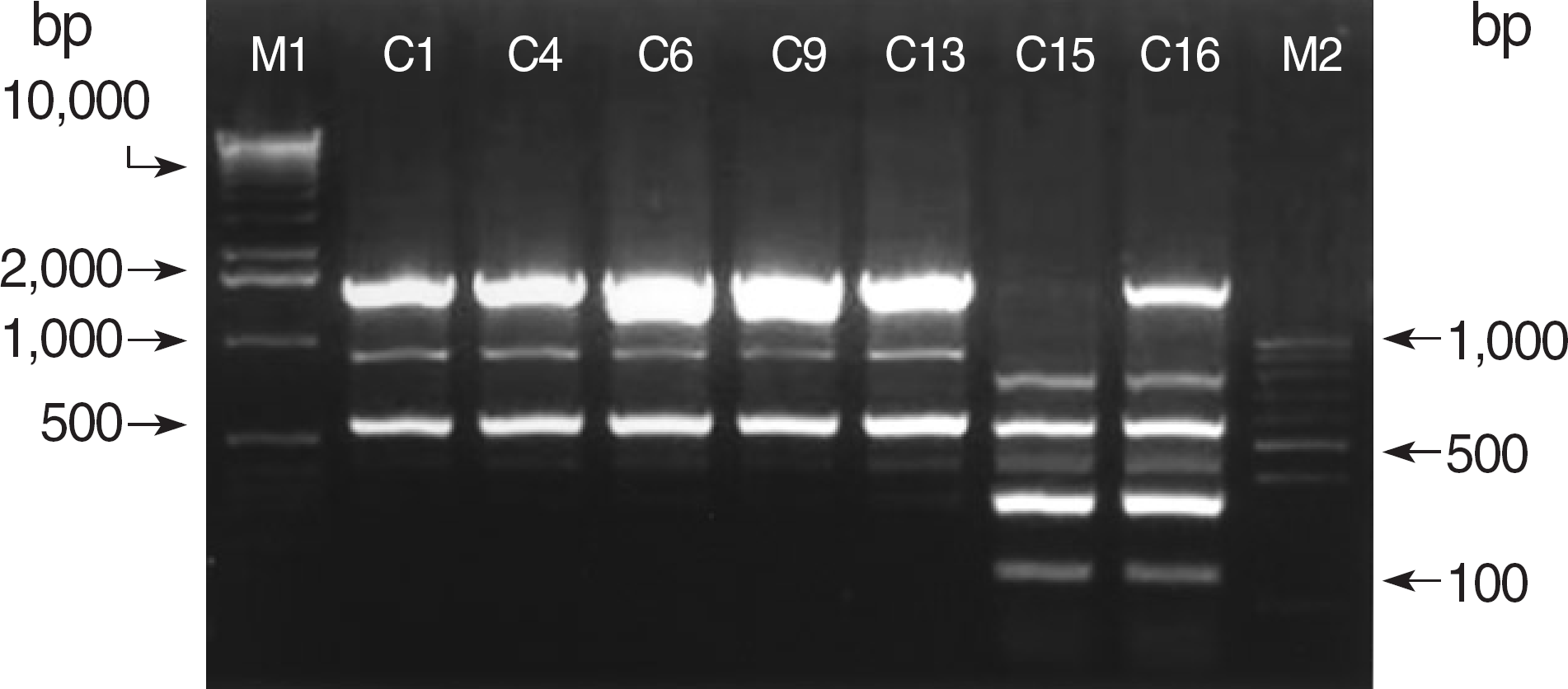
Fig. 2.
ERIC-PCR patterns of genomic DNA from ten clinical isolates of A. baumannii harboring blaIMP-1. Lane M1 is a 1 kb DNA size marker.
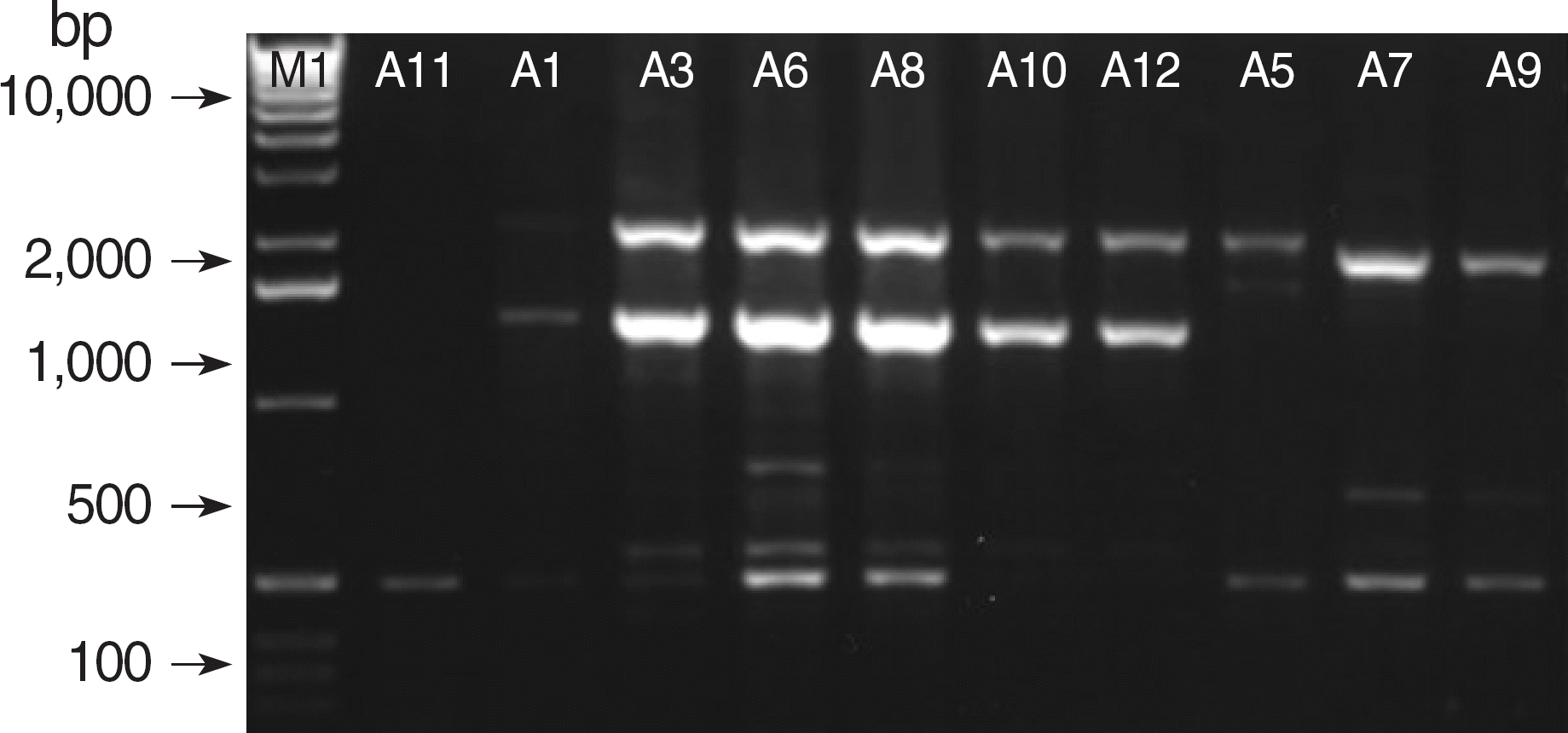
Table 1.
Oligonucleotides used as primers for amplification and sequencing in this study
Table 2.
Characterization of carbapenem-resistant A. baumannii isolates
| Isolates | Antibiotic susceptibilities | Clover-leaf | IPD | β-lactamase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | GEN | TOB | ATM | CAZ | FEP | IPM | MEM | PIP | TZP | CIP | ||||
| A1 | R | R | R | R | R | R | R | R | R | I | R | + | + | IMP-1, OXA-2 |
| A3 | R | R | R | R | R | R | R | R | R | I | R | + | + | IMP-1, OXA-2 |
| A4 | R | R | R | R | R | R | R | R | R | I | R | - | - | |
| A5 | S | S | I | I | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| A6 | S | R | I | R | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| A7 | R | R | R | R | R | R | R | R | R | I | R | + | + | IMP-1, OXA-2 |
| A8 | S | R | R | R | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| A9 | R | R | R | R | R | R | R | R | I | S | R | + | + | IMP-1, OXA-2 |
| A10 | S | R | R | R | R | R | R | R | R | R | R | + | + | IMP-1, OXA-2 |
| A11 | S | S | I | R | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| A12 | S | R | R | R | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| A14 | R | R | R | R | R | R | R | R | R | R | R | - | - | |
| A15 | S | S | S | R | R | R | R | R | R | R | S | - | - | |
| A16 | S | R | R | R | R | R | R | R | R | R | I | - | - | |
| B25 | R | R | R | I | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| B75 | R | R | R | I | R | R | R | R | S | S | I | + | + | IMP-1, OXA-2 |
| B297 | R | R | R | I | R | R | R | R | R | S | S | + | + | IMP-1, OXA-2 |
| B814 | R | R | R | R | R | S | R | R | S | R | S | + | + | VIM-2, OXA-2 |
| B852 | R | R | R | I | R | I | R | R | R | S | I | + | + | IMP-1, OXA-2 OXA-23 |
| C1 | R | R | R | R | R | R | R | R | R | R | R | + | - | |
| C2 | R | R | R | R | R | R | R | R | R | I | R | + | + | IMP-1, OXA-2 |
| C3 | R | R | R | R | R | R | R | R | R | R | R | - | - | |
| C4 | R | R | R | R | R | R | R | R | R | R | R | + | - | OXA-23 |
| C5 | R | R | R | R | R | R | R | R | R | R | R | - | - | |
| C6 | R | R | R | R | R | R | R | R | R | R | R | + | - | OXA-23 |
| C9 | R | R | R | R | R | R | R | R | R | R | R | + | - | OXA-23 |
| C13 | R | R | R | R | R | R | R | R | R | R | R | + | - | OXA-23 |
| C14 | R | R | R | R | R | R | R | R | R | I | R | - | - | |
| C15 | R | R | R | R | R | R | R | R | R | R | R | + | - | OXA-23 |
| C16 | R | R | R | R | R | R | R | R | R | R | R | + | - | OXA-23 |
| C17 | R | R | R | R | R | R | R | R | R | I | R | - | - | |
| 19606∗ | - | - | ||||||||||||
| YMC† | + | + | VIM-2 | |||||||||||
Table 3.
Prevalence of β-lactamases in carbapenem- resistant A. baumannii isolates




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download