Abstract
Background
Nucleophosmin gene (NPM1) mutation may be a good molecular marker for assessing the clinical status and predicting the outcomes in AML patients. We evaluated the applicability of NPM1 type A mutation (NPM1-mutA) quantitation for this purpose.
Methods
Twenty-seven AML patients with normal karyotype but bearing the mutated NPM1 were enrolled in the study, and real-time quantitative PCR of NPM1-mutA was performed on 93 bone marrow (BM) samples (27 samples at diagnosis and 56 at follow-up). The NPM1-mutA allele burdens (represented as the NPM1-mutA/Abelson gene (ABL) ratio) at diagnosis and at follow-up were compared.
Results
The median NPM1-mutA/ABL ratio was 1.3287 at diagnosis and 0.092 at 28 days after chemotherapy, corresponding to a median log10 reduction of 1.7061. Significant correlations were observed between BM blast counts and NPM1-mutA quantitation results measured at diagnosis (γ=0.5885, P=0.0012) and after chemotherapy (γ=0.5106, P=0.0065). Total 16 patients achieved morphologic complete remission at 28 days after chemotherapy, and 14 (87.5%) patients showed a >3 log10 reduction of the NPM1-mutA/ABL ratio. The NPM1-mutA allele was detected in each of five patients who had relapsed, giving a median increase of 0.91-fold of the NPM1-mutA/ABL ratio at relapse over that at diagnosis.
Assessment of residual disease burden is important for AML patients who had received chemotherapy, because the residual disease status of these patients has been correlated with their clinical course [1234,5678]. Currently, flow cytometric residual disease monitoring, using specific aberrant expression markers found at diagnosis, reportedly has less clinical relevance in AML than in ALL [19,1011]. Specific cytogenetic abnormalities found in patients at AML diagnosis are good candidates as residual disease monitoring markers at patient follow-up. However, the frequency of cytogenetic abnormalities detected at AML diagnosis is approximately 55% [121314], which significantly hinders their use as a general residual disease monitoring method.
Molecular assessment of specific mutation burdens detected at diagnosis using real-time quantitative PCR (RQ-PCR) could be another candidate for residual disease monitoring in AML, especially in patients with a normal karyotype (NK) [1516171819], since the sensitivity of real-time PCR is higher than that of flow cytometry and conventional karyotyping. The frequently detected molecular aberrations in NK-AML include nucleophosmin (NPM1), Fms-related tyrosine kinase 3 internal tandem duplication (FLT3 ITD), and CCAAT/enhancer binding protein alpha (C/EBPα) mutations [202122]. Compared with FLT3 ITD and C/EBPα mutations, which have been reported to be relatively unstable during follow-up, the assessment of NPM1 mutation burden has been regarded to be more beneficial for the purpose of residual disease monitoring owing to its higher frequency in NK-AML and its demonstrated higher stability during follow-up [4567232425]. However, the comprehensive correlation analysis to evaluate the relationship between the NPM1 mutation burden and the results of BM assessment at diagnosis and follow-up in AML patients has not been widely performed. In the present study, we evaluated whether the NPM1 type A mutation (NPM1-mutA; TCTG insertion at the 960th nucleotide in exon 12) burden (as quantitated by RQ-PCR) correlates with the clinical status of AML patients bearing the allele mutation, and whether it could predict patient outcome, which would be useful in residual disease monitoring.
Twenty-seven NK-AML patients with NPM1-mutA (demonstrated by direct sequencing at Asan Medical Center from Jan 2008 to Dec 2009) were enrolled in the present study. A total of 93 bone marrow (BM) samples (27 at diagnosis and 56 at follow-up) were obtained retrospectively, with the median follow-up period of 15 months (range: 1-38 months). Among the 27 patients, five (18.5%) possessed FLT3 ITD mutations simultaneously at diagnosis. All patients received standard induction chemotherapy that consisted of cytarabine and daunorubicin as defined in the literature [26]. This regimen included the continuous intravenous infusion of 200 mg/m2/day cytarabine (100 mg/m2/day for patients >60 yr) on days 1-7 and 45 mg/m2/day daunorubicin on days 1-3. Morphologic complete remission (CR) was defined as the presence of <5% blasts and >20% cellularity in BM aspirates, obtained at 28 days after induction chemotherapy. Relapse was defined as the presence of >5% blasts in BM aspirates for patients who had previously achieved CR. A total of 16 (59.3%) patients achieved morphologic CR in the follow-up BM examination, whereas five (18.5%) patients experienced relapse during the follow-up periods. All relapsed patients possessed the FLT3 ITD mutation at diagnosis and showed variable results in the follow-up BM study performed at 28 days after diagnosis (two patients with persistent disease, two patients with hypocellular marrow, and one patient in morphologic CR). The study was approved by the Institutional Review Board of Asan Medical Center, and all patients provided written informed consent for the genetic analysis.
The quantitation of NPM1-mutA burden was performed by using the Real-Q NPM1-mutA Quantitation Kit (Biosewoom Inc., Seoul, Korea), which uses RQ-PCR (reverse transcription of extracted total RNA into cDNA and amplification of DNA by real-time PCR). Total 93 BM aspirates were obtained and the test was performed following the manufacturer's instructions. The Abelson gene (ABL) was used as a reference housekeeping gene, and the allele burden of NPM1-mutA was represented as a ratio of its copy number relative to the ABL copy number (NPM1-mutA/ABL).
The NPM1-mutA quantitation results obtained at diagnosis and at 28 days after chemotherapy were assessed in each patient and analyzed in relation to the clinical condition of the patient. In addition, for each patient, the BM blast counts measured at diagnosis and at 28 days after chemotherapy were correlated with their NPM1-mutA quantity. These results are summarized in Table 1. For the five patients who experienced relapse during follow-up, NPM1-mutA mRNA levels at each follow-up time point before the relapse were assessed, and compared with the patient's clinical condition. These results are illustrated in Fig. 1.
The Spearman's correlation analysis was performed to see the correlation between the BM blast counts and NPM1-mutA quantitation results measured at diagnosis and at 28 days after chemotherapy. All tests were two-tailed, and P≤0.05 was considered significant. All analyses were performed by using SPSS 13.0.1 for Windows (SPSS Inc., Chicago, IL, USA).
The median NPM1-mutA/ABL ratio was 1.3287 at diagnosis and 0.0920 at 28 days after chemotherapy. When converted to log scale, the median log10 reduction at 28 days after chemotherapy relative to that at diagnosis was 1.7061. Spearman's correlation analysis results demonstrated significant positive correlation between the BM blast counts and NPM1-mutA quantitation results measured at diagnosis (γ=0.5885, P= 0.0012) and at 28 days after chemotherapy (γ=0.5106, P= 0.0065).
Of the 27 patients, 16 showed morphologic CR, seven developed hypocellular marrow, and four showed cancer persistence in the follow-up BM examination performed at 28 days after chemotherapy. Of the patients with morphologic CR, 13 patients showed complete loss of NPM1-mutA burden, and the additional one patient showed log10 reduction values of 3.5162 at 28 days after chemotherapy. This means that 87.5% of patients achieved reduction of the NPM1-mutA/ABL ratio of >3 log10 at the morphologic CR state compared with that at diagnosis. Of the seven patients with hypocellular marrow, two patients showed complete loss of NPM1-mutA burden but the remaining five patients showed residual NPM1-mutA burden at 28 days after chemotherapy. All patients with cancer persistence showed persistent NPM1-mutA burden at 28 days after chemotherapy (Table 1).
In summary, 81.3% of patients with morphologic CR and 28.6% of patients with hypocellular marrow at 28 days after chemotherapy achieved complete loss of NPM1-mutA burden at that time. In contrast, 18.7% of patients with morphologic CR, 71.4% of patients with hypocellular marrow, and 100.0% of patients with persistence at 28 days after chemotherapy showed residual NPM1-mutA burden at that time.
Of the five patients who experienced relapse at follow-up, one patient (dark blue line) had complete loss of NPM1-mutA burden at the morphologic CR state, but showed increased burden at relapse. A second patient (light blue line) showed persistent NPM1-mutA burden during the persistence state, followed by reduced burden at the post BM transplantation state, and finally prominent increase of the NPM1-mutA burden at relapse. A third patient (purple line) showed complete loss of NPM1-mutA burden at the hypocellular marrow state but recorded marked increase of the NPM1-mutA/ABL ratio at relapse. The remaining two patients (green and red lines, respectively) recorded complete loss of mutation burden at the morphologic CR, hypocellular marrow, and post BM transplantation states during follow-up, but showed an increased NPM1-mutA/ABL ratio at relapse. All five relapsed patients had simultaneous NPM1-mutA and FLT3 ITD mutations at diagnosis, but showed loss of the FLT3 ITD mutation while retaining stable NPM1-mutA at relapse.
In summary, NPM1-mutA disappeared in almost all the patients at the morphologic CR, hypocellular marrow, and post BM transplantation states during follow-up, but appeared again with high stability at relapse. This represented a median mutation burden increase of 0.91-fold (range: 0.33-46.88) over that at diagnosis (Fig. 1).
The assessment of cancer remission using specific molecular markers in AML patients has been included in the treatment response criteria, with residual disease monitoring being regarded as an important tool in the management of these patients. Previous studies showed that the NPM1 mutation burden is correlated with the clinical status of patients and can be used for the prediction of relapse risk in AML patients with the mutated NPM1 gene [67]. The advantages of NPM1 mutation as a residual disease monitoring marker lie in its high stability at relapse and its relatively high incidence in NK-AML patients [4567232425262728]. Focused on this point, in the present study, we evaluated the applicability of NPM1-mutA quantitation by RQ-PCR for assessing the clinical status and predicting the outcomes of AML patients bearing this mutation.
We found that 81.3% of patients in morphologic CR and 28.6% with hypocellular marrow at 28 days after chemotherapy had achieved complete loss of NPM1-mutA, whereas 18.7% of patients in morphologic CR, 71.4% with hypocellular marrow, and 100.0% with cancer persistence at 28 days after chemotherapy showed residual NPM1-mutA burden at that time. In addition, we identified the prediction rate of morphologic CR at 28 days after chemotherapy to be 87.5% when a >3 log10 reduction of NPM1-mutA burden was recorded at that time compared with the value at diagnosis. These results suggest that NPM1-mutA burden indeed correlates with the clinical status of patients, and a >3 log10 reduction of NPM1-mutA burden is a reliable molecular marker for predicting morphologic CR at 28 days after chemotherapy. This underscores the need for NPM1-mutA quantitation in the clinical setting for the evaluation of residual disease burden if the patient harbors this gene mutation. Subsequent analysis also demonstrated the association between a complete loss of NPM1-mutA burden and morphologic CR, hypocellular marrow, and post BM transplantation status, and the high stability of this mutation at relapse. All these results point to the main conclusions that the quantity of NPM1-mutA correlates well with the patient's status categorized as CR, relapse, or persistence, as defined by the BM blast percentage, and that the increase in NPM1-mutA burden can predict the patient outcome. This supports the high validity of adopting the NPM1-mutA quantitation assay in the clinical setting, which corresponds to previous study conclusions [672728].
In the present study, the BM blast counts and NPM1-mutA levels had significantly positive correlation (P<0.05), both at diagnosis and at 28 days after chemotherapy. These results underscore our main observation above, that the NPM1-mutA level would be correlated with the patient's clinical status as categorized by BM blast counts (CR, persistence, or relapse). However, our study also revealed a wide range of NPM1-mutA levels among the 27 patients at diagnosis, with some patients showing a high mutation burden with high BM blast count and others showing a low mutation burden. The wide range of BM blast counts detected among our patient cohort may contribute to these results, and partial expression of mutated NPM1 protein in the BM blasts may be another possible explanation for the variations.
Although 12 patients had persistent NPM1-mutA burden at 28 days after diagnosis, only five patients experienced relapse at follow-up. We speculate that a small portion of residual leukemic blasts (<5%) would survive in the morphologic CR or hypocellular marrow state, and complete loss of NPM1-mutA burden may not be achieved in these patients. We found that the eight patients who were in the morphologic CR or hypocellular marrow state, but did not achieve complete loss of NPM1-mutA burden, possessed relatively higher BM blast counts at 28 days after diagnosis (3.4-4.2%) than the 14 patients who achieved complete loss of the gene mutation (0.8-2.4%), supporting our speculation above.
Besides monitoring of the residual disease burden, clarification of the association between the degree of reduction of NPM1-mutA burden and the clinical improvement of patients would provide important information to clinicians. Although our study found a relatively smaller log10 reduction of NPM1-mutA burden (range: 0.7790-2.2075) in patients with cancer persistence at 28 days after chemotherapy than in patients with morphologic CR (complete loss in 81.3% of patients, and 1.6348, 1.3514, and 3.5162 log10 reductions in the remaining three patients) and hypocellular marrow (complete loss in 28.6% of patients, and 2.2364, 1.7774, 2.4557, 2.5754, and 1.6220 log10 reductions in the remaining five patients), the statistical significance of the results could not be determined owing to the small patient cohort, and so our study could not evaluate this issue accurately. This would be the major limitation of this study and should be assessed in a future comprehensive study.
In conclusion, we demonstrated that the NPM1-mutA burden in NK-AML patients correlates well with their cancer status, with high stability at relapse, and could predict their outcomes. This emphasizes the need for including the NPM1-mutA quantitation assay in the treatment program of AML patients harboring this gene mutation.
Acknowledgments
This study was supported by the Medical Research Institute Grant (2010-2), Pusan National University Hospital.
References
1. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010; 115:453–474. PMID: 19880497.

2. Stentoft J, Pallisgaard N, Kjeldsen E, Holm MS, Nielsen JL, Hokland P. Kinetics of BCR-ABL fusion transcript levels in chronic myeloid leukemia patients treated with STI571 measured by quantitative real-time polymerase chain reaction. Eur J Haematol. 2001; 67:302–308. PMID: 11872078.

3. Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013; 122:1813–1821. PMID: 23847197.

4. Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood. 2009; 114:2220–2231. PMID: 19587375.

5. Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009; 27:5195–5201. PMID: 19752335.

6. Krönke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011; 29:2709–2716. PMID: 21555683.

7. Shayegi N, Kramer M, Bornhäuser M, Schaich M, Schetelig J, Platzbecker U, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013; 122:83–92. PMID: 23656730.
8. Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012; 120:2826–2835. PMID: 22875911.
9. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102:83–87. PMID: 21171509.
10. Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008; 111:3941–3967. PMID: 18198345.
11. Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995; 9:1783–1786. PMID: 7564526.
12. Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DK, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010; 28:2674–2681. PMID: 20439644.
13. Grimwade D. The clinical significance of cytogenetic abnormalities in acute myeloid leukaemia. Best Pract Res Clin Haematol. 2001; 14:497–529. PMID: 11640867.
14. Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004; 18:115–136. PMID: 15010150.
15. Paietta E. Minimal residual disease in acute myeloid leukemia: coming of age. Hematology Am Soc Hematol Educ Program. 2012; 2012:35–42. PMID: 23233558.
16. Kern W, Haferlach C, Haferlach T, Schnittger S. Monitoring of minimal residual disease in acute myeloid leukemia. Cancer. 2008; 112:4–16. PMID: 18000811.
17. Falini B, Martelli MP, Pileri SA, Mecucci C. Molecular and alternative methods for diagnosis of acute myeloid leukemia with mutated NPM1: flexibility may help. Haematologica. 2010; 95:529–534. PMID: 20378574.
18. Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010; 116:354–365. PMID: 20385793.
19. Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999; 341:1051–1062. PMID: 10502596.
20. Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012; 366:1079–1089. PMID: 22417203.
21. Ostronoff F, Othus M, Lazenby M, Estey E, Appelbaum FR, Evans A, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol. 2015; 33:1157–1164. PMID: 25713434.
22. Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005; 352:254–266. PMID: 15659725.
23. Gorello P, Cazzaniga G, Alberti F, Dell'Oro MG, Gottardi E, Specchia G, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006; 20:1103–1108. PMID: 16541144.
24. Alpermann T, Schnittger S, Eder C, Dicker F, Meggendorfer M, Kern W, et al. Molecular subtypes of NPM1 mutations have different clinical profiles, specific patterns of accompanying molecular mutations and varying outcome in intermediate risk acute myeloid leukemia. Haematologica. 2016; 101:e55–e58. PMID: 26471486.
25. Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016; 374:422–433. PMID: 26789727.
26. Park SH, Chi HS, Cho YU, Jang S, Park CJ. CEBPA single mutation can be a possible favorable prognostic indicator in NPM1 and FLT3-ITD wild-type acute myeloid leukemia patients with intermediate cytogenetic risk. Leuk Res. 2013; 37:1488–1494. PMID: 24054719.
27. Dvorakova D, Racil Z, Jeziskova I, Palasek I, Protivankova M, Lengerova M, et al. Monitoring of minimal residual disease in acute myeloid leukemia with frequent and rare patient-specific NPM1 mutations. Am J Hematol. 2010; 85:926–929. PMID: 20981679.
28. Palmisano M, Grafone T, Ottaviani E, Testoni N, Baccarani M, Martinelli G. NPM1 mutations are more stable than FLT3 mutations during the course of disease in patients with acute myeloid leukemia. Haematologica. 2007; 92:1268–1269. PMID: 17768124.
Fig. 1
Quantitation of the NPM1 type A mutation in bone marrow aspirates from five relapsed AML patients, obtained at diagnosis and at each follow-up day. The results for each patient are represented with different colored lines (dark blue, Patient No. 5; red, Patient No. 6; green, Patient No. 8; light blue, Patient No. 23; purple, Patient No. 27, respectively as in Table 1).
Abbreviations: NPM1-mutA, nucleophosmin mutation type A; ABL, the Abelson gene; CR, complete remission; Hypo, hypocellular marrow; BMT, bone marrow transplantation.
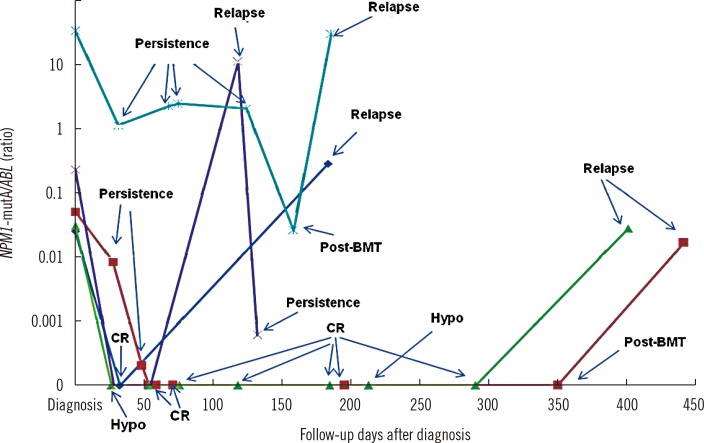
Table 1
Quantitation of NPM1 type A mutation burden in bone marrow aspirates of normal karyotype AML patients, at diagnosis and at 28 days after induction chemotherapy
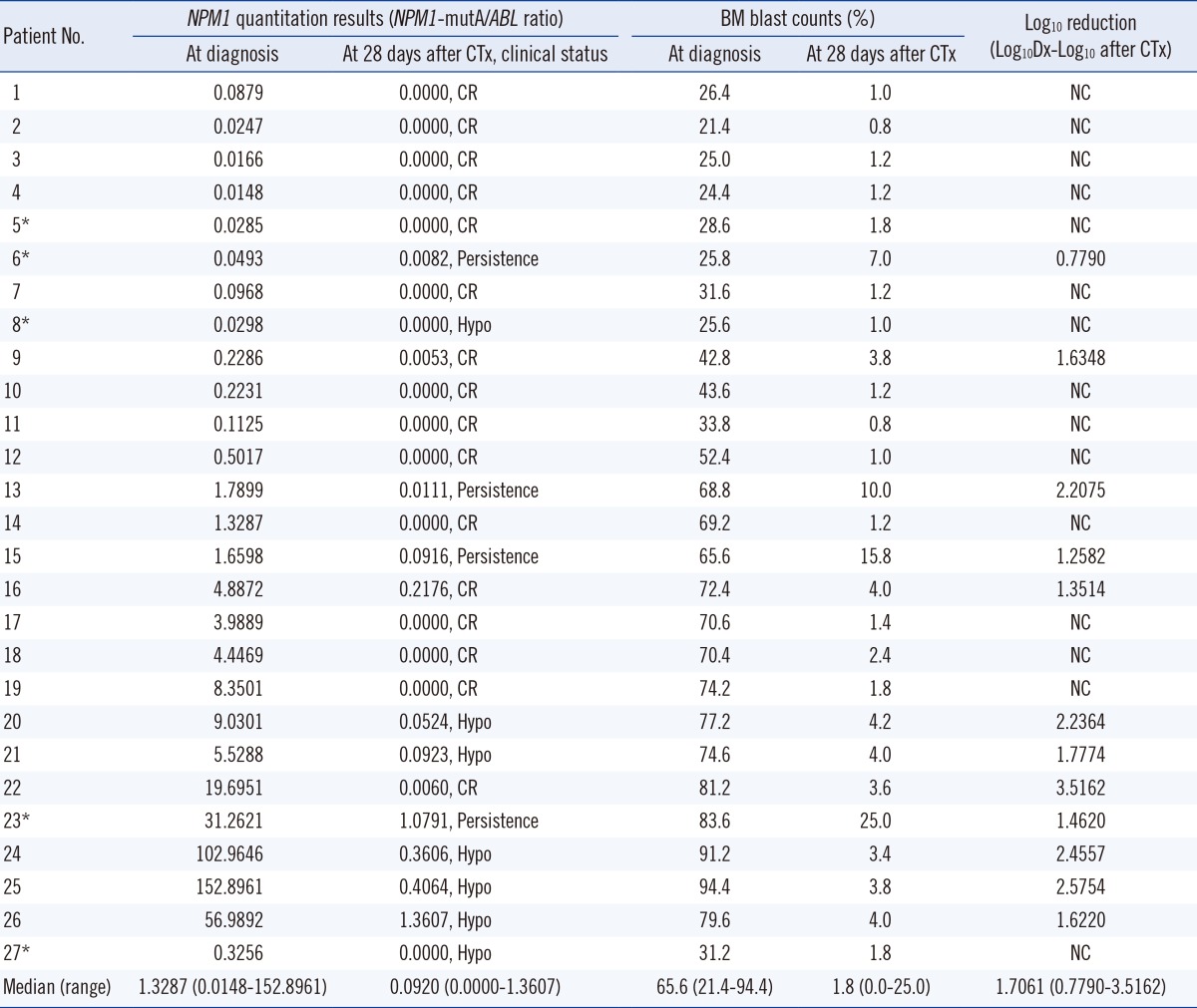
*The five patients with a simultaneous Fms-related tyrosine kinase 3 internal tandem duplication (FLT3 ITD) mutation at diagnosis, who demonstrated relapse at the follow-up periods.
Abbreviations: NPM1, nucleophosmin gene; mutA, mutation type A; ABL, the Abelson gene; CTx, chemotherapy; Dx, diagnosis; CR, complete remission; Hypo, hypocellular marrow; BM, bone marrow; NC, not calculated.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download