Abstract
Background
Angiotensin II type 1 receptor (AT1R) is responsible for cardiovascular effects mediated by angiotensin II. This study aimed to investigate the impact of antibodies directed against AT1R (anti-AT1R) in renal allograft rejection.
Methods
We evaluated 53 patients who had biopsy-proven rejection including antibody-mediated rejection (AMR) (N=22), T-cell-mediated rejection (TCMR) (N=29), and mixed AMR and TCMR (N=2). Donor specific HLA antibodies (DSA) and anti-AT1Rs were simultaneously determined.
Results
Anti-AT1Rs were detected in 9.4% (5/53) of rejection patients (one with acute AMR, two with chronic active AMR, one with acute TCMR, and one with mixed acute AMR & TCMR). HLA antibodies and DSA were detected in 75.5% (40/53) and 49.1% (26/53) of patients, respectively. There was no significant difference in transplant characteristics between anti-AT1R(+) and anti-AT1R(-) patients except for the association of HLA class-I DSA(+) and anti-AT1R(+). Four of five anti-AT1R(+) patients had DSA and were also found to have AMR. A single anti-AT1R(+)/DSA(-) patient developed acute TCMR. Detection rates of DSA, HLA antibodies, or anti-AT1R were not different between AMR and TCMR. However, DSA(+)/anti-AT1R(+) was more frequently found in AMR than in TCMR (P=0.036). Patients with anti-AT1R showed a greater tendency to develop high-grade rejection as Banff IIA/IIB or AMR.
Substantial developments in immunosuppressive therapy and HLA antibody detection have increased overall graft survival and reduced acute rejection [1]. Advantages in detecting HLA sensitization with the luminex bead assay have improved antibody-mediated rejection (AMR) prediction. The incidence of T-cell mediated rejection (TCMR) has decreased, and AMR is more frequently observed [1]. AMR is responsible for a large proportion of early and late allograft losses. However, up to 20% of patients without antibodies develop chronic allograft dysfunction, and non-HLA allo- and autoantibodies are increasingly recognized as a component of the immune response [2].
Anti-angiotensin II type 1 receptor activating antibodies (anti-AT1Rs) are associated with renal allograft rejection [1, 3, 4, 5, 6, 7, 8] and have been shown to stimulate severe vascular rejection [3]. AT1R is a seven transmembrane spanning G-protein coupled receptor that mediates most of the physiologic functions of the endogenous ligand, angiotensin II, including arterial blood pressure, and water-salt balance [4]. Autoantibodies to AT1R have been initially associated with hypertension in preeclampsia and malignant hypertension [6]. Anti-AT1R plays a role in autoreactive and alloreactive responses via multiple pathways. Anti-AT1Rs may develop through similar pathways to those observed for HLA specific antibodies, such as transfusions, pregnancies, and prior transplant. Anti-AT1Rs may also develop after tapering of immunosuppressive drugs or as a result of non-compliance [4]. While anti-AT1R has been reported as a risk factor for graft loss in association with acute rejection episodes, data on the incidence and pathologic association of anti-AT1R in patients with biopsy-proven rejection are currently lacking. In this study, we measured anti-AT1R in renal allograft rejection patients and investigated the incidence and clinical impact of anti-AT1R at the time of rejection.
We included 53 kidney-transplant recipients who had biopsy-proven renal allograft rejection between May 2011 and March 2013 at Seoul St. Mary's hospital, Seoul, Korea. Of the 53 patients, 22 patients had AMR, 29 patients had TCMR, and two patients had mixed AMR and TCMR. Serum samples were obtained at the time of biopsy to be screened for donor specific HLA antibodies (DSA) and were stored at -80℃ until anti-AT1R testing. Patients with pretransplant DSA or cross match-positivity were desensitized according to our center's desensitization protocol [9]. Briefly, rituximab at a dose of 375 mg/m2 (MabTheraTM; Genentech, San Francisco, CA, USA) was administered 7-10 days before transplantation, and plasmapheresis/immunoglobulin (100 mg/kg) therapy was administered every other day for 1-3 weeks with fresh-frozen plasma or albumin replacement fluids. The typical immunosuppression treatment regimen at our center has previously been described [9, 10]. Briefly, tacrolimus or cyclosporine was administered in combination with mycophenolatemofetil and prednisolone. Basiliximab was administered as induction therapy. In patients with DSA, the main regimen always comprised tacrolimus. In five anti-AT1R(+) patients, past history of hypertension and administration of AT1R-blocker during rejection episode were investigated. This study was approved by the institutional review board of Seoul St. Mary's Hospital.
Serum anti-AT1R levels were retrospectively assessed using the EIA-AT1R kit (One Lambda, Canoga Park, CA, USA). Microtiter 96-well polystyrene plates were coated with AT1R and in line with the manufacturer's instructions, 100 µL of diluted samples were incubated at 2-8℃ for 2 hr. After washing, plates were incubated for 60 min with 100 µL of horse radish peroxidase-labeled anti-human IgG. After incubation with 100 µL of tetramethylbenzidine substrate for 20 min, optical absorbance of each well was measured at 450 nm by using an ELISA microplate reader. Concentrations of anti-AT1R were determined by using a calibration curve, in which 10 U/mL was considered to be the cut-off value, in line with the manufacturer's recommendation.
Crossmatch tests were performed as previously described [11]. For complement-dependent cytotoxicity (CDC) testing, donor T- and B-cells were isolated by using CD19 monoclonal antibody attached to beads. For T-cell CDC, T-cell CDC-antihuman globulin (AHG), and B-cell CDC, 1 µL of donor T- or B-cell suspension (2×106 cells/mL) was incubated with 1 µL of recipient serum for 30 min at room temperature (RT), 60 min at RT, and 60 min at 37℃, respectively. Rabbit complement was added, and samples were incubated for 60 min, 120 min and 120 min at RT for T-cell CDC, T-cell CDC-AHG, and B-cell CDC, respectively. For T-cell CDC-AHG, after incubation of cells with serum, cells were washed and AHG 1 µL was added. Cells were then stained with acridine orange and ethidium bromide and observed for cytotoxicity by using an immunofluorescent microscope. CDC results were considered positive, when cell death exceeded that of the negative control well by 20%. If either T-cell CDC or CDC-AHG was positive, it was presented as T-CDC-positive. For flowcytometric crossmatch (FCXM) testing, 2×105 donor lymphocytes were added to 50 µL of patient serum and then incubated for 30 min at RT. Fluorescein isothiocyanate-labeled F(ab')2 anti-human IgG, specific for gamma-chain (Dako, Glostrup, Denmark), and phycoerythrin (PE)-labeled CD19 or CD3 (Dako) were added and incubated for 30 min. After washing, cells were analyzed using a Coulter EPICS XL (Beckman Coulter, San Diego, CA, USA). A positive FCXM was defined as a displacement of the mean channel fluorescence (MCF) relative to a negative control and donor autologous control. We also confirmed positive cases as having a relative median fluorescence (test MCF/[recipient autologous MCF+donor autologous MCF+healthy autologous MCF]/3) ≥1.5 and a test MCF greater than that of the negative MCF+ 3SDs.
For the detection of HLA antibodies and DSA, the Lifecodes LSA Class-I and II Single Antigens kit (Gen-Probe Transplant Diagnostics Inc., Stamford, CT, USA) was used. Microbeads in this kit are coated with over 90 different recombinant HLA-A, HLA-B, and HLA-C class-I antigens, and over 60 recombinant HLA-DRB, HLA-DQB, and HLA-DPB class-II antigens. According to the manufacturer's instructions, microbeads coated with purified HLA class-I and class-II glycoproteins were incubated with 10 µL of serum in 96-well plates for 30 min. After washes, the beads were incubated with 50 µL of 1:10 diluted R-PE-conjugated goat anti-human IgG for 30 min. A bead was considered positive, if two or more of the adjusted values were above the 1,000 median fluorescence intensity (MFI) cutoff on the Luminex 200 platform (Luminex Corp., Austin, TX, USA). If the detected HLA antibody corresponded to the mismatched donor HLA antigen, we defined the detected HLA antibody as DSA-positive. DSA class-I includes antibodies against donor HLA-A, B, and C, and DSA class-II includes antibodies against donor HLA-DR and DQB. DSA against HLA-DQB was determined on the basis of Korean DR-DQB linkage disequilibrium patterns [12].
Renal biopsy was performed when patients' serum creatinine level exceeded 120% of their baseline value. Rejection was diagnosed histologically according to the revised Banff 2007 classification [13]. To detect C4d deposition, indirect immunofluorescence staining was performed by using monoclonal antibodies against complement protein C4d (Biogenesis, Poole, UK; dilution 1:50). C4d positivity was defined as diffuse (>50%) and linear staining of peritubular capillaries.
Statistical analyses were performed with MedCalc software version 12.4.0.0 (MedCalc, Mariakerke, Belgium). The between-group differences of results were compared using chi-square test or independent samples T-test. The results are expressed as mean±standard deviation. All P values were two-tailed and values <0.05 were considered statistically significant.
Of 53 renal allograft rejection patients, 40 (75.5%) had HLA antibodies and 26 (49.1%) had DSA. Anti-AT1R was detected in 5 (9.4%) patients among 53 renal allograft rejection patients. Patient characteristics are described in Table 1. There was no significant difference in transplant characteristics between groups positive and negative for anti-AT1R, except for the presence of HLA class-I DSA. HLA class-I DSAs were found more frequently in anti-AT1R(+) patients than in anti-AT1R(-) patients (80.0% vs. 12.5%, P=0.003). However, there was no relationship between HLA class-II DSA detection and anti-AT1R positivity. More severe cases of Banff IIA/IIB or AMR were observed in four of five (80%) anti-AT1R(+) patients compared with 24/48 (50.0%) anti-AT1R(-) patients, but this difference was not statistically significant.
Anti-AT1Rs were detected in patients with acute AMR (N=1), chronic active AMR (N=2), acute TCMR (N=1), and mixed acute AMR and TCMR (N=1). Laboratory and clinical characteristics are summarized in Table 2. The levels of anti-AT1R were 12.1±3.1 (10.1-17.5) U/mL. Four of five anti-AT1R-positive patients had class-I or class-II DSA. Two patients (patients no. 3 and 5) developed post-transplant de novo DSA; in one further patient (patient no. 2), de novo DSA was suspected but could not be confirmed. Four patients who had both DSA and anti-AT1R revealed AMR on biopsy. A single patient who was anti-AT1R(+)/DSA(-) developed acute TCMR. Three of four anti-AT1R(+)/AMR(+) patients showed C4d deposition on their rejected allografts. Two of these patients were diagnosed as having chronic active AMR, and the third was diagnosed as having mixed TCMR with AMR. One patient with C4d-negative AMR (patient no. 4) had positive crossmatches and DSA with moderate MFI level before transplantation. Four months postoperatively, C4d-negative AMR was diagnosed at the protocol biopsy. At the time of biopsy, DSA level was low (MFI 2,000) and anti-AT1R was detected. This patient sustained a clinically stable allograft until 20 months after kidney transplantation.
Two of five anti-AT1R-positive patients had past history of hypertension, and one of them used AT1R-blocker during the rejection episode. None of the patients developed new onset malignant hypertension.
We analyzed HLA antibodies, DSA, and anti-AT1R results in association with histological rejection classification (AMR vs. TCMR) (Fig. 1). HLA antibodies and DSA data were also analyzed according to HLA class specificity. Two patients with AMR and TCMR mixed rejection were categorized as AMR. Of 24 patients with AMR, 20 patients showed C4d deposition, 11 patients had DSA, and four patients had both DSA and anti-AT1R. Of 13 patients who developed AMR and no DSA at the time of rejection, none had anti-AT1R. The detection rate of anti-AT1R, DSA, DSA class-I, DSA class-II, HLA antibodies, anti-HLA class I, or anti-HLA class II was not different between AMR and TCMR in patients with allograft rejection. However, C4d deposition and detection of both DSA and anti-AT1R were more frequent in AMR than in TCMR (P<0.001 for C4d deposition and P=0.036 for both DSA and anti-AT1R, respectively). MFI values of DSA class I or class II were not different between patients with AMR and TCMR (Fig. 2).
This study aimed to assess the incidence and role of anti-AT1R in renal allograft rejection patients. In this study, the frequency of anti-AT1R detection at the time of rejection was 9.4% in 53 renal transplant patients. Although sampling time is different, this percentage is lower than that of a previous study, which found that 71.4% of AMR patients had pre-transplant anti-AT1R >10 U/mL [1]. Nevertheless, we demonstrated the association between non-HLA anti-AT1R and HLA class-I DSA in renal allograft rejection patients, which is consistent with previous studies [1, 3, 4, 5, 6, 7, 8]. We also found that the detection rate of both anti-AT1R and DSA was higher in AMR than in TCMR (P=0.036), confirming anti-AT1R as a possible additive immunologic risk factor [1, 7]. A previous study with 351 patients revealed that patients with both anti-AT1R and DSA had lower graft survival than those with DSA alone [7]. In a pediatric renal recipient, a synergistic effect of high titered anti-AT1R and class-II DSA was also observed, and contributed to an accelerated rejection and hypertensive encephalopathy [8]. Amico et al. [14] reported a similar synergic effect between DSA and anti-AT1R, showing that four of six patients with both DSA and anti-AT1R lost their allograft, a higher rate of rejection than in patients with DSA alone. In our study, HLA-class I DSAs were more frequently detected in patients with anti-AT1R(+) and patients with anti-AT1R positivity developed more severe cases of Banff IIA/IIB or AMR. This finding supports the previous report that anti-AT1R may exacerbate the effects of DSAs, resulting in stronger immune responses [15].
Reinsmoen et al. [6] reported a strong association between the presence of high levels of pre-transplant anti-AT1R (>17 U/mL) and AMR in recipients whose sera contained no DSA or major histocompatibility class-I chain-related gene A (MICA). In this study, of five anti-AT1R(+) patients, only one with chronic active AMR had a high concentration of anti-AT1R (17.5 U/mL) at the time of rejection. Although we could not compare pre- and post-transplant levels of anti-AT1R, our findings support those of a previous study that found anti-AT1R concentrations at the time of rejection to be lower than pre-transplant levels [1]. A similar phenomenon is associated with intra-graft antibody adsorption of HLA antibodies, and the observed changes in anti-AT1R could be mediated by a similar mechanism [16].
Although evidence is accumulating for the existence of C4d-negative AMR, the deposition of C4d in vessels of the grafts serves as a good marker for AMR diagnosis [6]. In our study, 20 of 24 (83.3%) AMR cases showed C4d deposition. In terms of anti-AT1R, IgG1 and IgG3 subclasses are responsible for fixing complement [5]. However, in a previous study, C4d was detected in only 5 of 16 anti-AT1R positive patients who had no DSA [5]. Similarly, Reinsmoen et al. [6] reported that C4d staining was present in only one of six AMR patients who had high levels of anti-AT1R and no DSA. They suggested that the pathogenesis of rejection by anti-AT1R might be distinct from that by DSA. In the present study, three anti-AT1R-positive AMR patients showed C4d deposition on renal biopsy, but they also had DSA. One patient (patient no. 4) who had anti-AT1R and low DSA levels developed C4d-negative AMR. Therefore, the association between anti-AT1R and C4d stain cannot be confirmed in our study.
Previous studies reported that in 2-10% of patients without DSA, pre-transplant anti-AT1R antibodies were associated with AMR [5, 6, 14, 17]. However, in our study, using results at the time of rejection, no anti-AT1R positivity was found in 13 AMR patients without DSA. This may be due to the different sampling time, low sample volume, or skewed selection of patients. To assess the incidence of AMR attributable to anti-AT1R(+)/DSA(-), further studies with pre- and post-transplant sera will be needed.
In our study, the single patient who was AT1R (+) and DSA (-) developed acute TCMR. Giral et al. [1] reported that one-third of anti-AT1R(+) patients displayed no DSA and approximately 40% of patients with TCMR had anti-AT1R. A possible mechanism by which anti-AT1R leads to TCMR has been described: anti-AT1R may amplify local inflammation, which increases antigen expression and the production of Th1 cytokines and inflammatory chemokines, which may stimulate TCMR [4, 5, 18].
Hypertension can enhance the expression of HLA class-II antigens in renal transplants and increase the exposure of anti-AT1R to the recipient's immune system [19]. In a previous report, high-titer anti-AT1Rs were associated with rapid-onset malignant hypertension and encephalopathy during the rejection period [5]. In our study, two of five anti-AT1R-positive patients had pre-transplant hypertension, and none of them developed new onset malignant hypertension. These findings are consistent with a previous report, [6] which showed that most high levels of AT1R are not associated with hypertensive nephrosclerosis but are instead due to a variety of other diseases. This finding also supports the hypothesis that center-related differences in early post-transplant antihypertensive treatment may greatly contribute to the variability in hypertensive responses across different studies [5, 14, 17].
In this study, we investigated anti-AT1R only at the time of biopsy in allograft rejection patients. Other potential limitations of our study are the small number of allograft rejection patients, lack of randomization without long term follow-up data, and no standardized treatment protocol. It might be difficult to definitively prove the role of anti-AT1R separately from DSA on the basis of this study. Nevertheless, our study focused on anti-AT1R levels at the time of rejection and provided valuable information on the association between AMR and anti-AT1R.
In conclusion, the presence of anti-AT1R was significantly associated with HLA class-I DSA in renal allograft rejection patients, and both anti-AT1R and DSA positivity were associated with AMR in patients who developed renal allograft rejection. Further prospective studies with a large number of recipients would be needed to confirm the immunologic risk of anti-AT1R in renal transplantation.
Acknowledgments
This study was supported by Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea.
References
1. Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013; 13:2567–2576. PMID: 23919486.

2. Cox ST, Stephens HA, Fernando R, Karasu A, Harber M, Howie AJ, et al. Major histocompatibility complex class I-related chain A allele mismatching, antibodies, and rejection in renal transplantation. Hum Immunol. 2011; 72:827–834. PMID: 21664940.

3. Banasik M, Boratyńska M, Kościelska-Kasprzak K, Kamińska D, Bartoszek D, Zabińska M, et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int. 2014; 27:1029–1038. PMID: 24909812.

4. Reinsmoen NL. Role of angiotensin II type 1 receptor-activating antibodies in solid organ transplantation. Hum Immunol. 2013; 74:1474–1477. PMID: 23831255.

5. Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005; 352:558–569. PMID: 15703421.

6. Reinsmoen NL, Lai CH, Heidecke H, Haas M, Cao K, Ong G, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010; 90:1473–1477. PMID: 21030904.

7. Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013; 13:2577–2589. PMID: 23941128.

8. Kelsch R, Everding AS, Kuwertz-Bröking E, Brand E, Spriewald BM, Sibrowski W, et al. Accelerated kidney transplant rejection and hypertensive encephalopathy in a pediatric patient associated with antibodies against angiotensin type 1 receptor and HLA class II. Transplantation. 2011; 92:e57–e59. PMID: 22067217.

9. Chung BH, Choi BS, Oh EJ, Park CW, Kim JI, Moon IS, et al. Clinical impact of the baseline donor-specific anti-human leukocyte antigen antibody measured by Luminex single antigen assay in living donor kidney transplant recipients after desensitization therapy. Transpl Int. 2014; 27:49–59. PMID: 24118413.

10. Kim Y, Park KH, Chung BH, Choi BS, Yang CW, Kim JI, et al. Pretransplant IFN-γ ELISPOT assay as a potential screening test to select immunosuppression protocols for patients receiving basiliximab induction therapy. Transl Res. 2012; 160:230–236. PMID: 22683414.

11. Jang JY, Kim YJ, Kim Y, Park YJ, Han K, Oh EJ. Application of calculated panel reactive antibody using HLA frequencies in Koreans. Ann Lab Med. 2012; 32:66–72. PMID: 22259781.

12. Chung HY, Yoon JA, Han BY, Song EY, Park MH. Allelic and haplotypic diversity of HLA-A, -B, -C, and -DRB1 genes in Koreans defined by high-resolution DNA typing. Korean J Lab Med. 2010; 30:685–696. PMID: 21157157.

13. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760. PMID: 18294345.

14. Amico P, Hönger G, Bielmann D, Lutz D, Garzoni D, Steiger J, et al. Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation. 2008; 85:1557–1563. PMID: 18551059.

15. Reinsmoen NL, Lai CH, Mirocha J, Cao K, Ong G, Naim M, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014; 97:595–601. PMID: 24162250.

16. Martin L, Guignier F, Mousson C, Rageot D, Justrabo E, Rifle G. Detection of donor-specific anti-HLA antibodies with flow cytometryin eluates and sera from renal transplant recipients with chronic allograft nephropathy. Transplantation. 2003; 76:395–400. PMID: 12883199.
17. Scornik JC, Guerra G, Schold JD, Srinivas TR, Dragun D, Meier-Kriesche HU. Value of posttransplant antibody tests in the evaluation of patients with renal graft dysfunction. Am J Transplant. 2007; 7:1808–1814. PMID: 17524074.

18. Dragun D, Philippe A, Catar R. Role of non-HLA antibodies in organ transplantation. Curr Opin Organ Transplant. 2012; 17:440–445. PMID: 22790079.

19. Schindler R, Tullius SG, Tanriver Y, Noack K, Qun Y, Jürgensen JS, et al. Hypertension increases expression of growth factors and MHC II in chronic allograft nephropathy. Kidney Int. 2003; 63:2302–2308. PMID: 12753322.

Fig. 1
Detection rates of serum anti-AT1R, DSA, anti-HLA, and tissue C4d deposition in renal allograft rejection patients with AMR and TCMR. Two patients with AMR and TCMR mixed rejection were categorized as AMR.
Abbreviations: anti-AT1R, antibodies directed against AT1R; DSA, donor specific HLA antibodies; AMR, antibody-mediated rejection; TCMR, T-cell-mediated rejection.
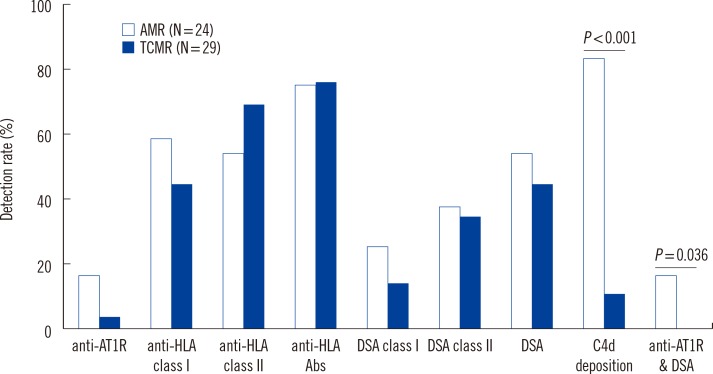
Fig. 2
Median fluorescence intensity values of detected DSA class I and class II were not different between patients with AMR and TCMR. The top and bottom border of the box means 95% confidence interval. The bars below and above the box mean minimum and maximum values, respectively, and horizontal line in the box means median value.
Abbreviations: DSA, donor specific HLA antibodies; AMR, antibody-mediated rejection; TCMR, T-cell-mediated rejection.
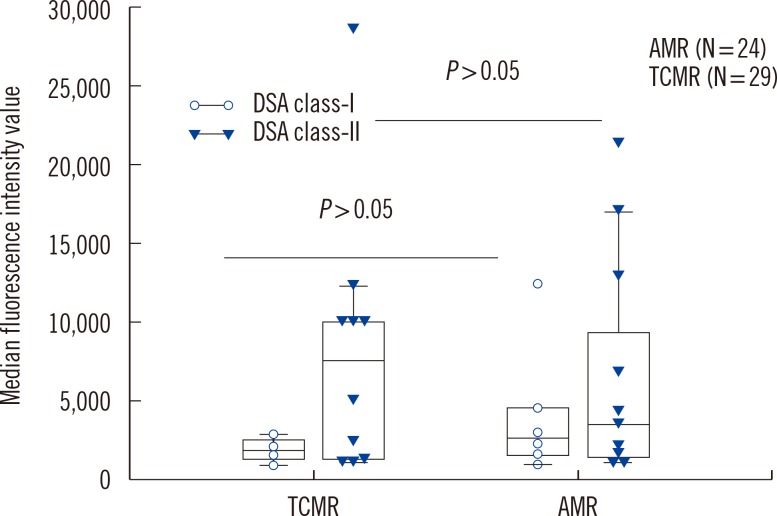
Table 1
Demographic characteristics of the study population according to the anti-AT1R results

Table 2
Laboratory and clinical characteristics of five renal allograft rejection patients with anti-AT1R-positive results

Abbreviations: N, negative; P, positive; NA, not available; M, male; F, female; Pt, patients; CDC, complement dependent cytotoxicity crossmatch; FCXM, flow cytometry crossmatch; Abs, antibodies; HTN, hypertension; ARB, angiotensin II receptor blocker; DSA, donor specific HLA antibodies; AMR, antibody-mediated rejection; TCMR, T-cell-mediated rejection; MM, mismatch; T/B, T cell/B cell.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download