Abstract
Background
Methods
Results
Figures and Tables
Fig. 1

Fig. 2

Fig. 3

Fig. 4

Table 1

*Variables are expressed as arithmetic mean±SD; †P values were <0.001 for all of the pairwise groups; ‡P=0.011 vs. group II, P=0.005 vs. group III, P<0.001 vs. group III and group IV; §P=0.001 vs. group II; ∥P=0.038 vs. group I, P=0.002 vs. group II; ¶P<0.001 vs. group I and group II; **P<0.001 vs. group I and group II; ††P=0.014 vs. group I, P=0.006 vs. group II; ‡‡P=0.02 vs. group I, P=0.002 vs. group II; §§P<0.001 vs. group I and group II; ∥∥P=0.03 vs. group I, P=0.006 vs. group II; ¶¶P<0.001 vs. group I and group II.
Abbreviations: OC, osteocalcin; ucOC, undercarboxylated osteocalcin; TACP, total acid phosphatase; ALP, alkaline phosphatase; BMD, bone mineral density.
Table 2

*Variables are expressed as median (25th-75th interquartile range); †P=0.026 vs. the normal group; ‡P=0.001 vs. the normal group, P=0.011 vs. the osteopenia group; §P=0.050 vs. the osteopenia group; ∥P=0.002 vs. the normal group, P=0.004 vs. the osreopenia group.
Abbreviations: BMD, bone mineral density; BMI, body mass index; OC, osteocalcin; ucOC, undercarboxylated osteocalcin; TACP, total acid phosphatase; ALP, alkaline phosphatase.
Table 3
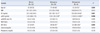
*Variables are expressed as median (25-75th interquartile range); †P=0.004 vs. the normal group; ‡P=0.031 vs. the normal group; §P=0.017 vs. the normal group; ∥P<0.001 vs. the normal group, P=0.004 vs. the osteopenia group; ¶P=0.021 vs. the normal group; **P=0.010 vs. the normal group; ††P=0.023 versus the normal group; ‡‡P=0.005 vs. the normal group, P=0.038 vs. the osteopenia group.
Abbreviations: BMD, bone mineral density; BMI, body mass index; OC, osteocalcin; ucOC, undercarboxylated osteocalcin; TACP, total acid phosphatase; ALP, alkaline phosphatase.
Table 4

*Variables are expressed as median (25-75th interquartile range); †P=0.007 vs. the normal group; ‡P=0.014 vs. the normal group; §P=0.029 vs. the normal group; ∥P<0.001 vs. the normal group, P=0.029 vs. the osteopenia group; ¶P=0.037 vs. the normal group; **P=0.004 vs. the normal group; ††P=0.034 vs. the normal group; ‡‡P=0.004 vs. the normal group, P=0.050 vs. the osteopenias group.
Abbreviations: BMD, bone mineral density; BMI, body mass index; OC, osteocalcin; ucOC, undercarboxylated osteocalcin; TACP, total acid phosphatase; ALP, alkaline phosphatase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download