Abstract
Objectives
The goal of this study was to define the radiologic characteristics of two-phase computed tomography (CT) of salivary gland Warthin tumors and to compare them to pleomorphic adenomas. We also aimed to provide a foundation for selecting a surgical method on the basis of radiologic findings.
Methods
We prospectively enrolled 116 patients with parotid gland tumors, who underwent two-phase CT preoperatively. Early and delayed phase scans were obtained, with scanning delays of 30 and 120 seconds, respectively. The attenuation changes and enhancement patterns were analyzed. In cases when the attenuation changes were decreased, we presumed Warthin tumor preoperatively and performed extracapsular dissection. When the attenuation changes were increased, superficial parotidectomy was performed on the parotid gland tumors. We analyzed the operation times, incision sizes, complications, and recurrence rates.
Results
Attenuation of Warthin tumors was decreased from early to delayed scans. The ratio of CT numbers in Warthin tumors was also significantly different from other tumors. Warthin tumors were diagnosed with a sensitivity of 96.1% and specificity of 97% using two-phase CT. The mean operation time was 38 minutes and the mean incision size was 36.2 mm for Warthin tumors. However, for the other parotid tumors, the average operation time was 122 minutes and the average incision size was 91.8 mm (P<0.05).
Preoperative prediction of the histopathologic characteristics of parotid gland neoplasms is essential in treatment planning, in estimating patient prognosis, and in particular for avoiding unnecessary surgery. Although various diagnostic methods are available, the preoperative diagnosis of parotid tumors remains challenging; the diagnostic accuracy of fine needle aspiration cytology (FNAC), the most important diagnostic test, is as low as 60%-80%. Computed tomography (CT) is usually performed as well as FNAC prior to surgical intervention. With the recent developments in CT technology, sufficient images from various phases can be obtained simultaneously through the use of multi-detector CT (MDCT). Methods to differentiate Warthin tumor from other tumor types using this CT technology have been described [1,2].
Superficial parotidectomy is widely performed to treat parotid tumors, with the aims of minimizing tumor cell spillage and preventing recurrence, because parotid tumors are usually located in the superficial lobe of the parotid gland [3,4]. However, it has been reported that treating Warthin tumors, which are well encapsulated and have a smooth surface, with extracapsular dissection was sufficient, because there were few differences in the recurrence rates with this procedure compared to the surgical outcomes of superficial parotidectomy [5,6,7,8].
The purposes of this study were to confirm the preoperative diagnosis of parotid gland Warthin tumors using two-phase MDCT and to assess the effectiveness of extracapsular dissection as a treatment for Warthin tumors. The operation times, incision sizes, complications, and recurrence rates were compared according to the surgical method used.
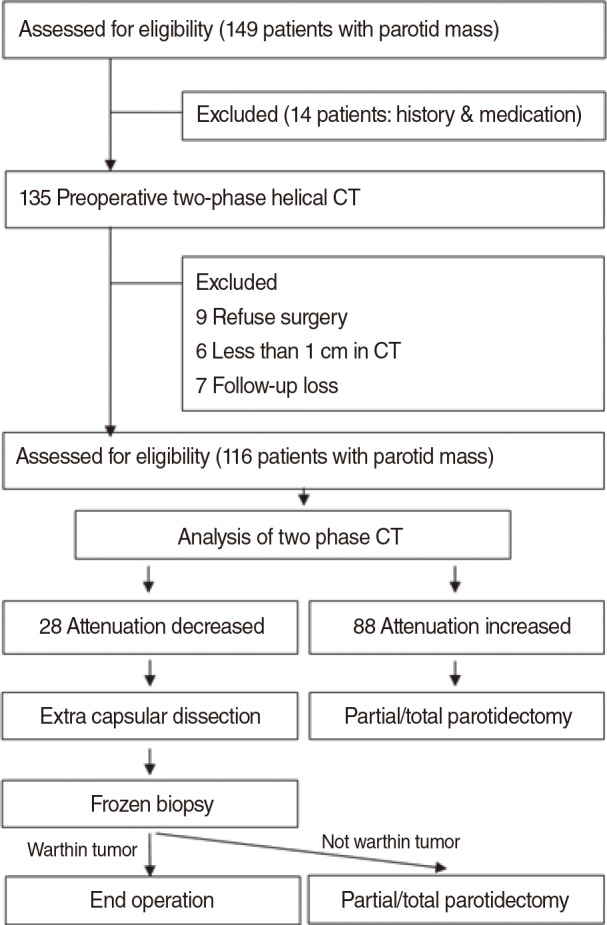
This was a prospective cohort analysis, and the study protocols were approved by Institutional Review Board of Gyeongsang National University Hospital before proceeding. Written informed consent was obtained from all participants. The study flowchart is presented in Fig. 1. From May 2011 to May 2012, 149 patients who presented to our outpatient clinic with parotid region tumors were initially enrolled in the study. Only patients older than 20 years of age who underwent surgery for parotid masses with the long axis longer than 1 cm (as measured by CT) were included. Patients with previous histories of head and neck malignancy, radiation therapy in the head and neck area, medications affecting salivary gland function, parotitis, or other medical problems detected by presurgical tests that could affect the pathological diagnosis of Warthin tumor or other parotid tumors were excluded from the study. Of the 149 initially enrolled patients, 33 did not meet the eligibility criteria and were excluded from the study.
Finally, 116 patients (75 males, 41 females; age range, 26 to 74 years; mean age, 45.2 years) who underwent preoperative two-phase helical CT scans (HiSpeed Advantage; GE Medical Systems, Milwaukee, WI, USA) and extracapsular dissection or partial/total parotidectomy were included in the analysis.
For helical CT scans, a total of 90 mL of iopamidol (Iopamiro 300; Bracco, Milan, Italy) was administered into an antecubital vein at a rate of 3 mL/sec using a power injector. Early and delayed phase scans were then obtained with scanning delays of 30 and 120 seconds, respectively. Scanning began at the skull base and continued toward the thoracic inlet with the following parameters: 35-second acquisition time, 5-mm collimation, and 5-mm/sec table speed. Contiguous transverse images were reconstructed from the volumetric data at 5-mm intervals. Thirty-five sections were obtained in each phase.
Each CT scan was evaluated separately by two different head and neck radiologists who were not aware of the results of the histopathologic analysis. The radiologists reached a consensus in every case in which there was a discrepancy in the interpretation of the images. First, the enhancement patterns of the tumors were visually assessed. Then, the attenuation change in the tumor between the early and delayed phases was analyzed and categorized as a "decrease" or "increase" in Hounsfield units (HU). Any obvious cystic or necrotic areas that consistently showed low attenuation in each phase were excluded from this assessment. The visual assessment of CT images in each phase was performed at the same window width (240 HU) and level (20 HU) in all patients.
Next, we quantitatively measured the CT numbers (in HU) of the tumors in each phase using circular regions of interest (ROIs). Each ROI was made as large as possible (8-30 mm2), and obvious cystic or necrotic areas were excluded. The ratio of delayed phase HU to early phase HU was also calculated for each patient.
In cases in which the attenuation change was decreased, we presumed that the parotid gland tumor was a Warthin tumor and performed extracapsular dissection. However, when the attenuation change was increased, we presumed the tumors were parotid gland tumors other than Warthin tumors and performed partial/total parotidectomy.
For the partial/total parotidectomy, the modified Blair incision was used, and the extracapsular dissection was performed with a superficial incision above the mass. Following extracapsular dissection, in cases in which the frozen section biopsy did not confirm a diagnosis of Warthin tumor, the operation was changed to partial/total parotidectomy (Fig. 1).
Phase analyses were conducted after classifying the cases into a Warthin tumor group and another tumor group. The diagnostic sensitivity, the specificity, the negative predictive value, and the positive predictive value were compared. The operation times, the incision sizes, and the complications experienced by the two groups were also compared. The waiting time for the result of the frozen section biopsy was not included in the operation time. All of the data were prospectively collected, and IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the statistical analyses. Robust analysis of variance (ANOVA) by Tamhane's T2 test was used to validate the significance. A P-value of <0.05 was considered significant.
A total of 116 patients with parotid tumors underwent surgical interventions. There were 75 male and 41 female patients, for a ratio of 1.8:1 (male:female). The average age of the subjects was 45.2 years old (range, 26 to 74 years), with the greatest percentage of patients in their 60s. The mean follow-up period was 12.3 months.
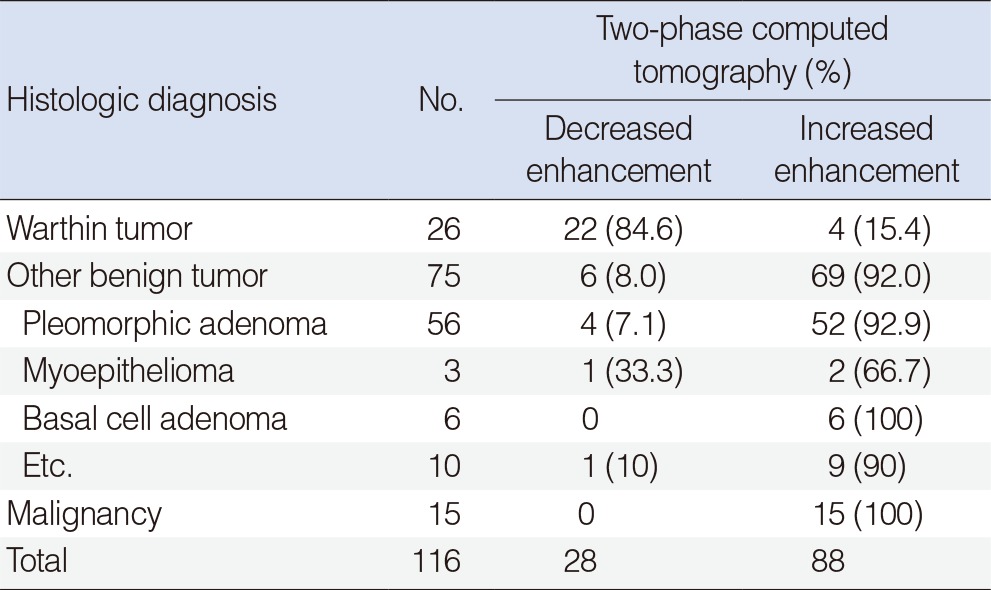
On visual assessment, delayed phase scans showed increased attenuation in 51/56 of pleomorphic adenomas (92%), in 14/15 of malignant tumors (93%), and in 69/75 of other benign tumors (92%) compared with early phase scans. However, 22/26 of Warthin tumors (84.6%) showed a decrease in attenuation on delayed phase scans (Table 1).
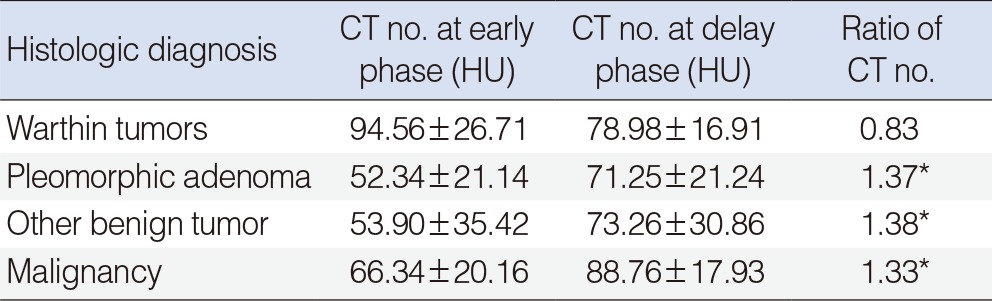
With quantitative measurement, the mean CT numbers on early phase scans were highest for Warthin tumors (94±26 HU), followed by malignant tumors (66±34 HU), other benign tumors (53±90 HU), and pleomorphic adenomas (52±21 HU). These differences in CT numbers between the various tumor types were statistically significant (P=0.005). However, there were no significant differences in mean attenuation between pleomorphic adenomas and malignant tumors or between pleomorphic adenomas and other benign tumors (P>0.05).
On delayed phase scanning, the mean CT numbers were 88±17 HU for malignant tumors, 71±21 HU for pleomorphic adenomas, 78±16 HU for Warthin tumors, and 73±30 HU for other benign tumors. There were no significant differences in mean attenuation between the histopathologic tumor types on delayed phase scanning.
Warthin tumors showed a characteristic decreasing enhancement pattern between the early phase and the delayed phase CT scans, while the other parotid tumors demonstrated an increase in enhancement (Table 1).
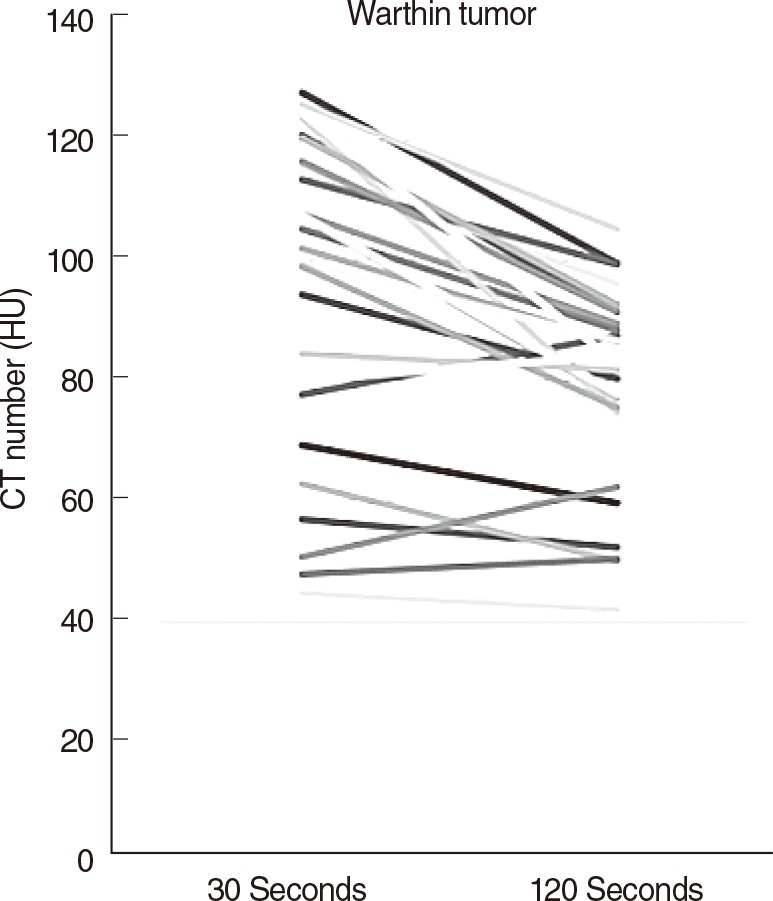
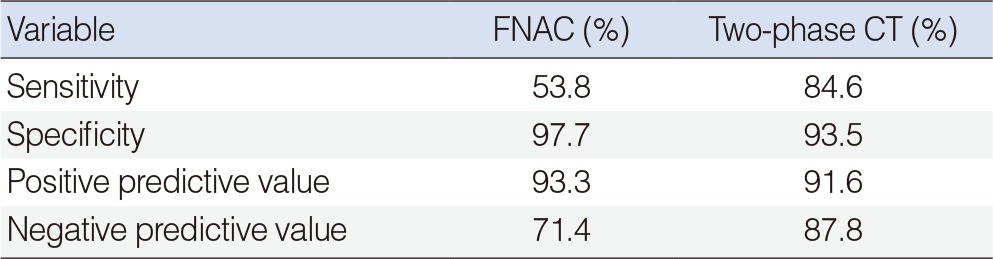
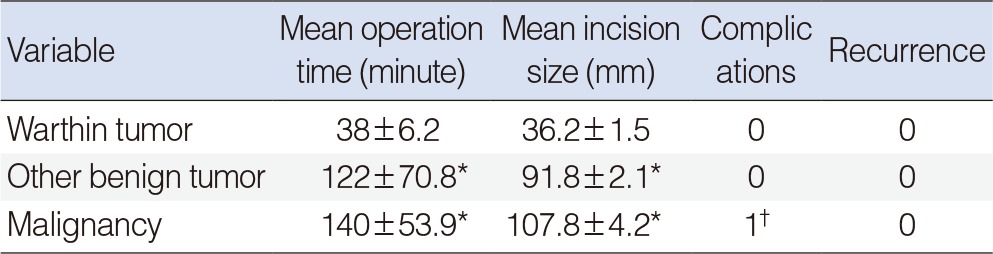
Twenty-six cases were finally confirmed to be Warthin tumor. There were 22 cases with decreased enhancement and 4 cases with increased enhancement. However, the enhancement increases were only less than 5 HU in 2 of the 4 cases with increased enhancement (Fig. 2). The sensitivity, the specificity, the positive predictive value, and the negative predictive value were 53.8%, 97.7%, 93.3%, and 71.4% when fine needle biopsy were solely considered and those when CT were solely considered were 96.1%, 93.5%, 92.5%, and 96.6%, respectively (Table 2). The average operation time was 38±6.2 minutes and the average incision size was 36.2±1.5 mm (Table 3). Some of the patient who underwent parotidectomy complained of postsurgical auricular hypoesthesia, but the patients who underwent extracapsular dissection did not any discomfort.
Seventy-five cases were finally diagnosed as other benign tumors. Among these tumors, increased enhancement was observed in 69 cases and decreased enhancement was seen in 6 cases. These 6 cases were confirmed to be pleomorphic adenoma (4 cases), myoepithelioma (1 case), and one lymph node. Parotidectomy was performed for 52 cases of pleomorphic adenoma with increased enhancement. Extracapsular dissection was initially performed for 4 cases with decreased enhancement, but these 4 cases were ultimately also removed by parotidectomy after frozen section biopsy revealed them to be pleomorphic adenoma. Among 19 other benign tumors, not including Warthin tumor and pleomorphic adenoma, the 18 cases with increased enhancement were removed by parotidectomy and the 1 case showing decreased enhancement was removed by extracapsular dissection because frozen section biopsy revealed that it was a myoepithelioma.
The average operation time was 122±70.8 minutes and the average incision size was 91.8±2.1 mm (Table 3). Some of the patients who underwent superficial parotidectomy complained of temporary postsurgical auricular hypoesthesia.
Increased enhancement was shown in all 15 instances of malignant tumors, and all of the tumors were removed by parotidectomy. The extent of operations was determined by the results of preoperative investigations and frozen section biopsies. Total parotidectomy was performed in 10 cases and cervical lymph node dissection was performed in 5 cases. The average operation time was 140±53.9 minutes and the average incision size was 107.8±4.2 mm. All patients complained of temporary postsurgical auricular hypoesthesia, and one patient developed a postsurgical hematoma, which was resolved by evacuation of the hematoma and hemostasis (Table 3).
Warthin tumor, also known as papillary cystadenoma lymphomatosum, was named by Warthin [9] in 1929. Histopathologically, Warthin tumor shows well encapsulated cystic spaces lined by a bilayered epithelium which resembles papillary architecture wrapped around abundant lymphocytes [10,11]. It is generally known as the second most common benign parotid tumor. It is also reported that 10% of the tumors have bilateral onset and 10%-30% have multiple onset. Warthin tumor is unlikely to advance to malignancy, but progression to malignancy has been reported in about 0.3% of cases [6,12]. The tumor is known to be prevalent among middle-aged men with a history of smoking.
FNAC is an effective method to examine salivary gland tumors, in particular. Its sensitivity and the specificity to differentiate malignancy have been reported to be 72.7% and 95.7% in studies conducted in South Korea [13]. The diagnostic rate for benign tumors is reported to be even higher, above 80% in some studies [14]. The typical characteristics of Warthin tumor in FNAC are the oncocytic epithelium and lymphoid stroma. However, Warthin tumor can be misdiagnosed as malignancy if there is squamous metaplastic change without the tumor's typical characteristics; therefore, other clinical features should also be considered [15].
On CT images, Warthin tumors are usually found in the superficial parotid gland. Cystic components and solid stroma exist together in most cases, and mildly increased enhancement is observed in the solid tissues. Unlike with other tumors, the peak enhancement is typically seen in Warthin tumors 30 seconds after injection of a contrast agent [1,2]. Two-phase CT was used in this study, and decreased enhancement was observed in 22 cases out of a total of 26 cases of Warthin tumor (84.6%), results similar to the findings of two previous studies. Comparing the phase enhancements among the three different groups of tumor types observed in the study, it was confirmed that Warthin tumor showed decreased enhancement in the delayed phase, unlike other types of benign tumors or malignant tumors. ANOVA testing also confirmed that there was a significant difference in the alteration ratio of enhancement between the Warthin tumor group and the other groups (Table 4). Among the 4 cases of Warthin tumor without decreased enhancement, 2 cases were mostly composed of cystic component, with very little solid stroma observed. For this reason, it was estimated that accurate HU values were not obtained in either the early or delayed phases. In the other 2 cases, there was increased enhancement, in contrast to the majority of Warthin tumors. This was thought to be because the enhancement was slowly increased and slowly decreased in Warthin tumors with a relatively low cellular density, resulting in an outcome unlike that in most Warthin tumors, which show a rapid increase of enhancement and a rapid washout due to the abundant small vessels that stain for CD31 and have a high extensive capillary network [16]. Therefore, this study suggests that two-phase CT can increase the diagnostic accuracy when attempting to differentiate Warthin tumor from other types of parotid tumors preoperatively.
Superficial parotidectomy, the operation to remove the entire portion of the parotid gland superficial to the facial nerve, is widely performed to prevent the recurrence of superficial parotid tumors and has been accepted as a safe surgical method since it was introduced by Comoretto and Barzan [3], Janes [4], and Bailey [17]. However, superficial parotidectomy may result in complications such as Frey syndrome, temporary or permanent facial paralysis, facial depressions, and aesthetic disturbances. A number of studies have been conducted on conservative surgical methods such as Extracapsular dissection and partial parotidectomy, with the aims of reducing both tumor recurrence and the occurrence of these complications [4,5,7,18,19]. Although the rate of recurrence can be reduced when some of the normal tissues around the tumor are removed along with the tumor by these conservative methods, the risks of residual tumor cells and incomplete removal of multiple tumors still remain. In addition, some warn that a lack of pathologic evidence can result in a suboptimal dissection [18]. Surgical excision is the primary choice in the treatment of Warthin tumor, but the ideal extent of the operation remains controversial. Warthin tumors are usually found under the superficial lobe of the parotid gland. On macroscopic examination, they can be seen as gray- or brown-colored cystic lesions covered with a well-developed capsule. It is also observed that they lack pseudopods, unlike pleomorphic adenoma. For these reasons, it has been reported in several studies that Warthin tumors can be safely and adequately removed by extracapsular dissection without altering the recurrence rate [5,6,7,8]. On the other hand, some claim that superficial parotidectomy is the minimal necessary operation because bilateral or multiple onsets are more common in Warthin tumor compared to other types of parotid tumors, while others insist that total parotidectomy is essential because the recurrence rate is still reported to be up to 25% when the condition is treated with superficial parotidectomy [20,21]. It has also been reported that some cases of recurrence were caused by undiscovered tumors rather than by incomplete dissection [22]. In the current study, Warthin tumors were treated with extracapsular dissection. Use of extracapsular dissection significantly reduced the incision size and the average operation time for Warthin tumors (Table 3).
Extracapsular dissection have risk of facial nerve injury because the facial nerve branches can be identified for operation, but parotidectomy also have that risk. The facial nerve injury can be avoided by facial nerve mornitoring device. Furthermore, any patients did not have facial nerve injury in this study. Although extracapsular dissection removed only the well-developed tumor capsules instead of also removing the normal tissues around the tumors, no instances of recurrence were reported. Due to the recent developments in CT technology, even the smallest parotid tumors were able to be identified, and no cases of recurrence caused by undiscovered tumors were reported either. Hence, it is judged that extracapsular dissection is a safe and effective method for the treatment of Warthin tumor. It is true that the average follow-up period of 12.3 months in this study is considered relatively short for the proper evaluation of recurrence, and close observation for a longer period of time will be required in future studies. Nevertheless, the effectiveness of extracapsular dissection has been confirmed, since this study observed statistically significant reductions in both the operation time and the incision size, as well as the absence of hypoesthesia of the ear, in patients who underwent the procedure.
Among parotid tumors, which are difficult to diagnose preoperatively, Warthin tumor could be diagnosed with two-phase CT with a sensitivity of 84.6% and a specificity of 93.5%. Decreased enhancement patterns on delayed phase scanning using two-phase CT can be helpful in establishing a diagnosis of WT, and extracapsular dissection can be performed as an effective treatment.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A1A1012542). This research was supported by Leading Foreign Research Institute Recruitment Program through the NRF funded by the Ministry of Education, Science and Technology (MEST) (2012K1A4A3053142).
References
1. Choi DS, Na DG, Byun HS, Ko YH, Kim CK, Cho JM, et al. Salivary gland tumors: evaluation with two-phase helical CT. Radiology. 2000; 1. 214(1):231–236. PMID: 10644130.

2. Yerli H, Aydin E, Coskun M, Geyik E, Ozluoglu LN, Haberal N, et al. Dynamic multislice computed tomography findings for parotid gland tumors. J Comput Assist Tomogr. 2007; Mar-Apr. 31(2):309–316. PMID: 17414771.

3. Comoretto R, Barzan L. Benign parotid tumour enucleation: a reliable operation in selected cases. J Laryngol Otol. 1990; 9. 104(9):706–708. PMID: 2172430.
4. Janes RM. The treatment of tumors of the salivary glands by radical excision. Can Med Assoc J. 1940; 12. 43(6):554–559. PMID: 20321924.
5. Yoo GH, Eisele DW, Askin FB, Driben JS, Johns ME. Warthin's tumor: a 40-year experience at The Johns Hopkins Hospital. Laryngoscope. 1994; 7. 104(7):799–803. PMID: 8022240.
6. Kwon KH, Suh JH, Hur MH, Chung WY, Kang HY, Park CS. Enucleation for the management of the parotid Warthin's tumor. J Korean Surg Soc. 2001; 11. 61(5):474–478.
7. Heller KS, Attie JN. Treatment of Warthin's tumor by enucleation. Am J Surg. 1988; 10. 156(4):294–296. PMID: 3177754.

8. Choi JO, Ju EJ, Kim WJ, Choi HY, Chu HR, Choi G, et al. Selection of surgical treatment for Warthin's tumors of parotid: experience in 20 cases. Korean J Otolaryngol-Head Neck Surg. 1999; 4. 42(4):501–504.
9. Warthin AS. Papillary cystadenoma lymphomatosum: a rare teratoid of the parotid region. J Cancer Res. 1929; 13:116–125.
10. Schlakman BN, Yousem DM. MR of intraparotid masses. AJNR Am J Neuroradiol. 1993; Sep-Oct. 14(5):1173–1180. PMID: 8237699.
11. Hwang BT, Sugihara K, Kawashima K, Yamashita S. Scanning electron microscopic study of Warthin's tumor. J Oral Pathol. 1987; 3. 16(3):118–123. PMID: 3114450.

12. Batsakis JG. Pathology consultation: melanotic neuroectodermal tumor of infancy. Ann Otol Rhinol Laryngol. 1987; Jan-Feb. 96(1 Pt 1):128–129. PMID: 3028235.
13. Park A, Kim HK, Kim DW, Jin SY, Lee DW. Fine needle aspiration cytology of the salivary gland: an analysis of 221 cases. Korean J Cytopathol. 1999; 12. 10(2):133–143.
14. Kim JY, Pae KH, Choi SH, Kim SY, Nam SY. Clinical values of fine needle aspiration biopsy in salivary gland diseases. Korean J Otolaryngol-Head Neck Surg. 2006; 6. 49(6):639–643.
15. Ballo MS, Shin HJ, Sneige N. Sources of diagnostic error in the fine-needle aspiration diagnosis of Warthin's tumor and clues to a correct diagnosis. Diagn Cytopathol. 1997; 9. 17(3):230–234. PMID: 9285198.

16. Woo SH, Choi DS, Kim JP, Park JJ, Joo YH, Chung PS, et al. Two-phase computed tomography study of warthin tumor of parotid gland: differentiation from other parotid gland tumors and its pathologic explanation. J Comput Assist Tomogr. 2013; Jul-Aug. 37(4):518–524. PMID: 23863526.
17. Bailey H. The treatment of tumours of the parotid gland with special reference to total parotidectomy. Br J Surg. 1940; 1. 28(111):337–346.

18. Tae K, Lee HS, Hong DK, Park HK, Cho SH, Lee SH, et al. Partial parotidectomy as a conservative procedure for the parotid tumor. Korean J Otolaryngol-Head Neck Surg. 2003; 7. 46(7):592–597.

19. Helmus C. Subtotal parotidectomy: a 10-year review (1985 to 1994). Laryngoscope. 1997; 8. 107(8):1024–1027. PMID: 9261001.

20. Zappia JJ, Sullivan MJ, McClatchey KD. Unilateral multicentric Warthin's tumors. J Otolaryngol. 1991; 4. 20(2):93–96. PMID: 2041071.
21. Gant TD, Hovey LM, Williams C. Surgical management of parotid gland tumors. Ann Plast Surg. 1981; 5. 6(5):389–392. PMID: 6264834.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download