Abstract
Hearing loss (HL) is one of the most frequent clinical manifestations of patients who suffer with multi-systemic genetic disorders. HL in association with other physical stigmata is referred to as a syndromic form of HL. LEOPARD syndrome (LS) is one of the disorders with syndromic HL and it is caused by a mutation in the PTPN11 or RAF1 gene. In general, 5 year old children who undergo cochlear implantation usually show a marked change in behavior regarding sound detection within the first 6 months of implant use, but word identification may not be exhibited for at least another 6-12 months of implant use. We herein report on a 5-year-old girl with LS. Her clinical manifestations including bilateral sensorineural HL, which indicated the diagnosis of LS. We confirmed the diagnosis by identifying a disease-causing mutation in the PTPN11 gene, which was a heterozygous missense mutation Ala461Thr (c.1381G>A). She underwent cochlear implantation (CI) without complications and she is currently on regular follow-up at postoperative 1 year. This is the first reported case of CI in a patient with LS in the medical literature.
Hearing loss (HL) is one of the most frequent clinical manifestations of patients with multi-systemic genetic disorders. The syndromic form of HL and HL in association with other physical stigmata accounts for 30% of the cases of hereditary HL. LEOPARD syndrome (LS) is one of the hereditary disorders that underlie syndromic HL and it is caused by a mutation in the PTPN11 gene. The acronym LEOPARD stands for the major features of the disorder: L, lentigines; E, electrocardiographic (ECG) conduction abnormalities; O, ocular hypertelorism; P, pulmonary stenosis; A, abnormalities of the genitalia; R, retardation of growth; and D, deafness (sensorineural) [1]. The disease is inherited in an autosomal dominant manner with variable penetrance and expressivity. Sporadic cases of LS have also been reported [2]. The diagnosis of LS is made based on clinical grounds, and molecular genetic analysis to detect disease-causing mutations is needed to confirm the diagnosis. The PTPN11 and RAF1 genes are the only genes currently known to be associated with LS, and a mutation in either gene is identified in more than 90% of the patients with LS [3-5]. Noonan syndrome (NS) is an allelic disorder of LS, and the clinical manifestations of NS significantly overlap with those of LS. In this report, we present our experience of cochlear implantation (CI) in a girl with LS. The diagnosis of LS was confirmed by the identification of a heterozygous missense mutation Ala461Thr in the PTPN11 gene.
A 5-year-old girl visited our hospital because of bilateral hearing loss. She was born at 32 weeks of gestation with a birth weight of 2,100 g via a vaginal delivery. On the newborn hearing screening test, she was diagnosed as having bilateral hearing loss. At 9 months of age, she had an operation for pulmonary stenosis. In the first year of life, dysorexia with feeding difficulty developed, which was caused by oromotor trouble, and this added to the development of ambulation disorder. Finally, she was diagnosed as having cerebral palsy and she has been maintained on rehabilitation therapy since then. The family history was not remarkable.
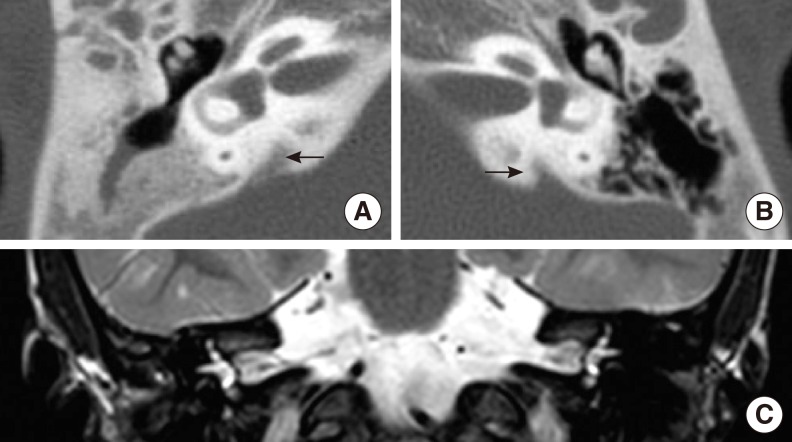
Physical examination revealed hypertelorism, a plane occiput and low-set ears (Fig. 1). She also had numerous symmetrically distributed dark brown macules involving the face, scalp, upper extremity, axilla and trunk. A transthoracic ECG for preoperative evaluation showed slightly increased left ventricular (LV) wall thickness with normal LV contractility. Any auditory brainstem response was not detected in either of the ears. The auditory steady-state response showed responses with 120 decibel (dB) at 1 kHz and 115 dB at 2 kHz on the left side, and 125 dB at 4 kHz on the right side. Based on the audiological evaluation as described above, she was diagnosed with bilateral profound hearing loss. Bilateral enlarged vestibular aqueducts (EVA) were identified on the computed tomography (CT) of the temporal bone and magnetic resonance imaging (MRI) of the internal auditory canal, but both the internal auditory canal were observed to be of normal size, as well as the cochlea and the semicircular canals, which were normally filled with fluid on both sides (Fig. 2).
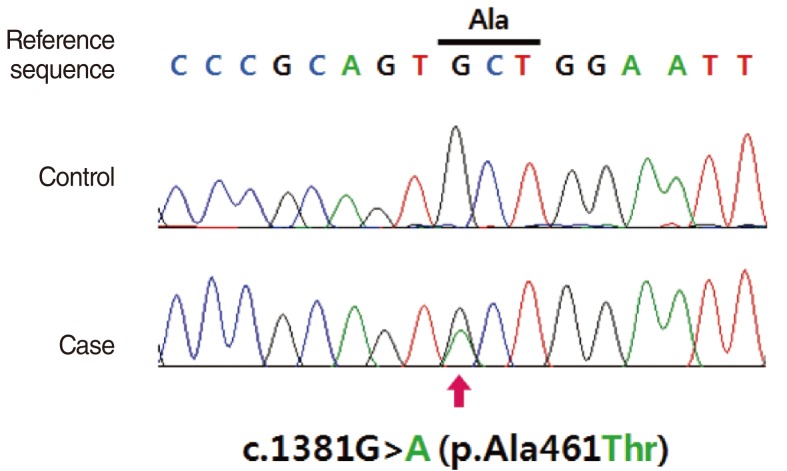
To investigate the etiology of the hearing loss and the other physical abnormalities of the patient, molecular study for the PTPN11 gene was performed based on the clinical diagnosis of LS. Informed consent was obtained from her parents. DNA was extracted from peripheral leukocytes according to the standard protocols. All the coding exons and intron-exon boundary sequences of the PTPN11 gene were bidirectionally sequenced using the Big Dye Terminator ABI Prism Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and the ABI PRISM 3100 Genetic Analyser (Applied Biosystems). As a result, the patient was found to be heterozygous for a missense mutation in exon 12. This mutation was a G-to-A transition at nucleotide position 1,381 and it was predicted to substitute the 461st residue alanine to threonine (c.1381G>A, p.Ala461Thr) (Fig. 3). The family study revealed that neither of the asymptomatic parents was carrying the mutation, indicating the de novo occurrence of the mutation.
The patient received CI in the left ear (Nucleus Freedom). The electrode was completely inserted in the scala tympani, and neural response telemetry (NRT) during the operation showed good results. The patient was able to respond to loud sound, but she still could not deliver meaningful communication on her last follow-up visit 1 year after CI, and her score on the CAP scale conformed to Category 1.
'Leopard' syndrome was first introduced in 1969 to describe the spotted appearance from lentiginosis [1]. The diagnostic criteria include multiple lentigines plus 2 other recognized features or a first-degree relative with multiple lentigines plus 3 other features in the proband [6]. Some of the clinical manifestations of LS, such as facial anomalies, distinct congenital heart defects, pectus deformities, hearing loss and growth retardation, are also observed in NS, which is a disorder that is genetically related to LS. Although skin pigmentary changes have been described in both disorders [3,7-9], the patients with NS are unlikely to have the profusion of pigmentary lesions, lentigines and café-au-lait patches or to be deaf.
The diagnosis of LS was made in our patient based on her clinical manifestations, including multiple lentigines, ocular hypertelorism, pulmonary stenosis and deafness. Lentigines are brown to black macules that are dispersed mostly on the face, neck and upper part of the trunk. Multiple lentigines are distinctive features of LS, although they may be absent in young patients. The patient in our case had numerous dark brown macules on the face and upper trunk, and this was compatible with multiple lentigines. ECG abnormalities, including progressive conduction anomalies, are the most common cardiac defects [10-12]. The patient's echocardiogram showed increased LV wall thickness, although there were no conduction abnormalities. Hypertrophic cardiomyopathy (HCM) is the most serious anomaly in patients with LS, and this can cause potentially life-threatening events and sudden death. HCM may progress and appear later in life; thus, cardiologic assessment at regular intervals was recommended to her parents due to the concern for the possibility of the progression of her LV wall thickness to HCM. Sensorineural deafness is the least common feature, which occurs in about 15%-25% of the patients with LS. Deafness (or HL) can be detected at birth, as in our patient, or during childhood; however, HL can also develop later in life [10,13].
Mutations in the PTPN11 gene are the genetic backgrounds of both LS and NS (PTPN11-related disorders). More recently, mutations in the RAF1 gene were reported in patients with LS [5]. The PTPN11 and RAF1 genes are both involved in the Ras pathway [5]. The PTPN11 gene encodes for the non-receptor protein tyrosine phosphatase SHP-2. It contains two Src homology 2 (SH2) domains and a protein tyrosine phosphatase (PTP) domain [4]. It is known that PTPN11 mutations causing LS/NS are gain-of-function mutations and they are most commonly in the form of missense (amino acid-changing) mutations [3]. The mutated amino acid in this patient, Ala461, was located in close proximity to Gly464 and Thr468 in exon 12, which is the tyrosine-specific protein phosphatase's active site (amino acids 457-469 [VHCSAGIGRTGTF]). The residue is necessary for PTP activity and this is a recurrent site of mutation in patients with LS, and particularly in association with HCM [3,13,14].
Early CI during the first year of life is important to achieve the best possible level of language acquisition in prelingual HL patients, given the establishment of a diagnosis and the proper indications. In general, a 5-year-old congenitally deaf child usually shows marked change in their behavior regarding sound detection within the first 6 months of implant use. However, word recognition may not develop for at least another 6-12 months of implant use [15]. But the benefit of CI in our patient has been minimal until post CI 1 year. It is possible that she concomitantly acquired cerebral palsy, which limits her ability to acquire language.
Before CI, the status of mastoid pneumatization and any associated inner ear anomalies should be evaluated with high resolution imaging modalities because structural anomalies of the inner ear and petrous bone have occasionally been reported in NS, which is a disorder that is genetically related to LS [16].
We describe here our experience of CI in a patient with LS from a PTPN11 mutation, and this is the first such report in the medical literature. Identification of the mutation in the PTPN11 gene would confirm the diagnosis of patients who are clinically suspected of having LS or NS. For a patient with LS combined with sensorineural hearing loss, audiologic tests should be performed at regular intervals, and hearing aids or performing CI should be considered in a timely manner.
ACKNOLWDEGMENTS
This study was supported by the Samsung Medical Center Clinical Research Development Program grant, #CRS-106-56-2.
References
1. Gorlin RJ, Anderson RC, Blaw M. Multiple lentigenes syndrome. Am J Dis Child. 1969; 6. 117(6):652–662. PMID: 5771505.

2. Seuanez H, Mane-Garzon F, Kolski R. Cardio-cutaneous syndrome (the "LEOPARD" syndrome): review of the literature and a new family. Clin Genet. 1976; 3. 9(3):266–276. PMID: 1261064.

3. Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002; 8. 71(2):389–394. PMID: 12058348.

4. Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002; 8. 39(8):571–574. PMID: 12161596.

5. Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007; 8. 39(8):1007–1012. PMID: 17603483.

6. Voron DA, Hatfield HH, Kalkhoff RK. Multiple lentigines syndrome: case report and review of the literature. Am J Med. 1976; 3. 60(3):447–456. PMID: 1258892.
7. Allanson JE, Hall JG, Van Allen MI. Noonan phenotype associated with neurofibromatosis. Am J Med Genet. 1985; 7. 21(3):457–462. PMID: 2411134.

8. Ahlbom BE, Dahl N, Zetterqvist P, Anneren G. Noonan syndrome with cafe-au-lait spots and multiple lentigines syndrome are not linked to the neurofibromatosis type 1 locus. Clin Genet. 1995; 8. 48(2):85–89. PMID: 7586657.
9. Colley A, Donnai D, Evans DG. Neurofibromatosis/Noonan phenotype: a variable feature of type 1 neurofibromatosis. Clin Genet. 1996; 2. 49(2):59–64. PMID: 8740913.

10. Coppin BD, Temple IK. Multiple lentigines syndrome (LEOPARD syndrome or progressive cardiomyopathic lentiginosis). J Med Genet. 1997; 7. 34(7):582–586. PMID: 9222968.

11. Limongelli G, Pacileo G, Marino B, Digilio MC, Sarkozy A, Elliott P, et al. Prevalence and clinical significance of cardiovascular abnormalities in patients with the LEOPARD syndrome. Am J Cardiol. 2007; 8. 100(4):736–741. PMID: 17697839.

12. Limongelli G, Sarkozy A, Pacileo G, Calabro P, Digilio MC, Maddaloni V, et al. Genotype-phenotype analysis and natural history of left ventricular hypertrophy in LEOPARD syndrome. Am J Med Genet A. 2008; 3. 146A(5):620–628. PMID: 18241070.

13. Sarkozy A, Conti E, Digilio MC, Marino B, Morini E, Pacileo G, et al. Clinical and molecular analysis of 30 patients with multiple lentigines LEOPARD syndrome. J Med Genet. 2004; 5. 41(5):e68. PMID: 15121796.

14. Sarkozy A, Obregon MG, Conti E, Esposito G, Mingarelli R, Pizzuti A, et al. A novel PTPN11 gene mutation bridges Noonan syndrome, multiple lentigines/LEOPARD syndrome and Noonan-like/multiple giant cell lesion syndrome. Eur J Hum Genet. 2004; 12. 12(12):1069–1072. PMID: 15470362.
15. Staller SJ, Beiter AL, Brimacombe JA. Use of the Nucleus 22 channel cochlear implant system with children. Volta Rev. 1994; 96:15–40.
Fig. 1
The patient had hypertelorism, ptosis and low-set ears (A), and diffuse lentiginosis on the anterior trunk (B).
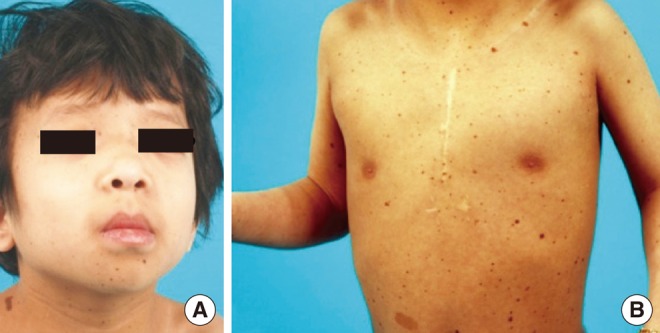
Fig. 2
Computed tomography (CT) of the temporal bone (A, B) and magnetic resonance imaging (MRI) of the internal auditory canal were performed (C). Bilateral enlarged vestibular aqueducts (arrow) were identified, but both of the internal auditory canals were observed to be of normal size. The cochlea and the semicircular canals were also of normal shape and filled with fluid on both sides. (A) temporal bone CT of the right side, (B) temporal bone CT of the left side, (C) T2 weighted MR coronal view image.




 Citation
Citation Print
Print


 XML Download
XML Download