Abstract
Objectives
Cartilage reshaping by laser irradiation is used to correct septal and auricular cartilage deformities. Chondrocyte viability following laser irradiation and reshaping has been well established. However, the regeneration process of chondrocyte after laser irradiation has not been revealed yet. The aims of this study were to determine the mechanism of cartilaginous thermal injury and the regenerative process of damaged cartilage following laser irradiation.
Methods
Laser irradiation was performed on human septal cartilage and rabbit auricular cartilage using a 1,460-nm diode laser. We observed change in the shape of cartilage and evaluated the extent of cartilage injury using live/dead cell assay via confocal microscopy. Hoechst and propidium iodide (PI) staining was used to evaluate the mechanism of chondrocyte injury after laser irradiation. To evaluate the regeneration of cartilage, laser irradiated cartilages were reimplanted into a subperichondrial pocket and were harvested at 1, 2, and 4 weeks after reimplantation for viability assessment and histologic examination.
Results
Laser irradiation using a 1,460-nm diode laser produced a marked shape change in both human septal and rabbit auricular cartilages. Thermal damage on cartilage was correlated with the exposure time and the laser power. Hoechst and PI staining showed that chondrocyte death by laser irradiation was due to mainly necrosis, rather than apoptosis. In lower power treatment group (0.3 W and 0.5 W), all the chondrocytes regenerated within 4 weeks, however, in 1 W treatment group, chondrocytes could not regenerate until 4 weeks.
Conclusion
Reshaping of cartilage using 1,460 nm diode laser was attained concurrently with the thermal injury to the chondrocytes. The extent of thermal damage on chondrocytes was dependent on the exposure time and the laser power and the damaged chondrocytes irradiated with lower level of laser power could be regenerated after reimplantation into subperichondrial pocket.
Laser cartilage reshaping (LCR), first introduced in 1993, is a novel technique designed to permanently alter the shape of cartilage [1]. It has been used in the field of reconstructive surgery for a number of cartilage deformities, and several studies have reported successful clinical application of LCR during septoplasty [2], otoplasty [3], and epiglottoplasty [4].
In spite of several successful reports, LCR has not been adopted widely in the clinical setting, since it is based on thermally induced internal relaxation of mechanically deformed cartilage. Heat generation by laser causes the injury to the chondrocytes, resulting in concurrent death of chondrocytes and significant shape changes of the cartilage. Due to this problem, several previous studies have focused on the potential thermal injury to the chondrocytes during photothermal heating to determine guidelines for safe and effective LCR [5-8]. However, few studies have evaluated the regeneration of damaged chondrocytes following laser irradiation, although this information is critically important to adapt LCR for use in the clinical field. Hence, we aimed to evaluate the degree and mechanism of thermal injury to cartilage, and the regenerative process of damaged cartilage after laser irradiation.
Human septal cartilage was obtained from 10 patients undergoing septal surgery. The average age was 23.8 ± 4.0 years (range, 18 to 30 years) with 7 men and 3 women. Harvested cartilage was carved to 1-2 mm thick with a shape of rectangle, and stored in normal saline solution at room temperature until use. Total 56 specimens were irradiated by 1,460 nm diode laser (CyT-ML1450, CyTroniQ, Cheonan, Korea) within 1 hour after harvest of the cartilage. Ethics approval was obtained from the Internal Review Board of the Dankook University Hospital.
Laser was delivered via an optic fiber (2 mm in spot diameter) in two different patterns: spot and linear. In spot pattern, the specimens were irradiated with laser powers of 0.5 W, 1.0 W, and 2.0 W for exposure time of 5, 10, and 20 seconds. In linear pattern, specimens were irradiated with laser powers of 0.5 W, 1.0 W, and 2.0 W in a to-and-fro manner for 10 and 20 seconds each.
Two separate 3×4 cm-sized segments of the auricular cartilage was harvested from six New Zealand white rabbits after elevating submucoperichondrial flap in each ear. The harvested cartilage was immediately divided into 5×10 mm sized rectangular slabs with a razor blade. More than 16 specimens were irradiated by 1,460 nm diode laser for 5 seconds at laser powers of 0.3 W, 0.5 W, and 1 W, respectively. The schematic diagram of experimental protocol was described in Fig. 1. These settings were decided after a preliminary study to obtain a range of laser parameters required to change the shape of the cartilage without significant thermal injury.
The average depth and width of the thermally damaged area were measured by a single observer who was unaware of the experimental conditions using embedded software in the Olympus microscope system.
The control and reshaped cartilage were reimplanted into a subperichondrial pocket of the rabbit ear. The quilting sutures were performed to provide fixation between perichondrium and reimplanted cartilages. Ceftizoxime was administered intramuscularly to prevent wound infection.
Cartilage specimens were reharvested at 1, 2, and 4 weeks after reimplantation. Subperichondrial planes were dissected carefully with iris scissors, and cartilages were harvested from the subperichondrial pockets and prepared for viability assessment and histologic examination (Fig. 1).
Cell viability assay were performed on the human and rabbit cartilage immediately after laser irradiation. The cartilages were sliced into 400 µm-thick-cross sections. They were stained with calcein acetoxymethylester (calcein AM) and ethidium homodimer-1 (EthD-1; Molecular Probes, Eugene, OR, USA). Calcein AM is an indicator of living cells that have intact cell membranes and esterase activity and Eth-D-1 is an indicator of dead cell. It can only permeate cells with compromised membranes. Stained specimens were inspected with a confocal microscope at 10× magnification (LSM 510 META, Carl Zeiss, Jena, Germany).
Harvested rabbit cartilages were examined at 1, 2, and 4 weeks after reimplantation by the same method to evaluate regeneration of damaged cartilage.
Hoechst and propidium iodide (PI) staining was used to evaluate the mechanism of thermal injury in rabbit cartilage after laser irradiation. Hoechst 33342 (Sigma-Aldrich Co., St Louis, MO, USA), a blue-fluorescence dye (excitation/emission maxima ≈350/461 nm when bound to DNA), stains the condensed chromatin in apoptotic cells more brightly than normal chromatin. PI, a red-fluorescence dye (excitation/emission maxima ≈535/617 nm when bound to DNA), is only permeant to dead cells. The staining pattern resulting from the simultaneous use of these 2 dyes makes it possible to distinguish normal, apoptotic, and necrotic dead cell populations by fluorescence microscopy.
The first stain (Hoechst solution, 4 µL) was added to 2 mL of medium. After incubating the sample in Hoechst solution overnight, the samples were rinsed with Dulbecco's phosphate-buffered saline for a few seconds. Then, the second stain (PI, 4 µL) was added to 2 mL of medium. Sample slides were finally examined by confocal microscopy (405 nm and 543 nm).
The remaining portions of the specimens were fixed in formalin and serially dehydrated using graded ethanol solutions and subsequently embedded in paraffin. Then, 6-µm sections were obtained, and stained with hematoxylin-eosin (H&E), and finally examined with a light microscope at 200× magnification.
Laser irradiation with given power and exposure time produced shape changes in all human septal cartilages without definite tissue ablation (Fig. 2). Cell viability test using live/dead assay showed a clear demarcation between live cells (green) and dead cells (red) in histology and the extent of the damaged area increased accordingly as the laser power and the exposure time increased (Fig. 3). The average depth and width of the thermally damaged area showed a linear correlation according to the laser power and the exposure time, irrespective of the irradiation method (spot or linear) (Fig. 3).
Laser exposure to rabbit auricular cartilage created a similar shape changes like in human septal cartilages. The extent of the damaged area in the rabbit auricular cartilage increased with the increase of the laser power (0.3-1.0 W) and the extent of dead chondrocytes in the 0.3 W and 0.5 W treatment groups was less extensive than in the 1 W treatment group (Fig. 4A). The depth of thermal damage showed no significant difference among treatment groups due to thin thickness of the auricular cartilage. However, the width of thermal damage increased from 736 µm in 0.3 W treatment group to 1,377 µm in 1 W treatment group (Table 1).
Hoechst and PI staining showed a dominance of red staining without blue fragmented nucleus-like an apoptotic body after laser irradiation, suggesting that cell death by laser irradiation resulted from mainly necrosis, rather than apoptosis (Fig. 4B).
In terms of regeneration capacity, we implanted irradiated cartilages in rabbit ear until 4 weeks. The angle of curvature had a tendency to recover to its original position over time and it reduced slightly at the time of harvest as compared with that immediately after irradiation. Chondrocytes in 0.3 W treatment group regenerated completely at 2 weeks and those in 0.5 W treatment group regenerated at 4 weeks, showing relationship between radiation power and recovery time. In 1 W treatment group, chondrocytes could not recover until 4 weeks (Fig. 5A). At 1 week after implantation, 75% of damaged chondrocytes in the 0.3 W treatment group regenerated. In 0.5 W treatment group, 33%, 73%, and 99% of damaged chondrocytes regenerated at 1, 2, and 4 weeks, respectively. In contrast, chondrocyte could not regenerate in 1 W treatment group until 4 weeks (Fig. 5B). Table 2 shows changes in the average depth and width of thermally damaged tissue. The width of the damaged area in the 0.3 W and 0.5 W laser groups decreased with time, while the width of the damaged area in 1 W laser group increased slightly.
H&E staining revealed loss of chondrocytes and condensation of nuclei in laser irradiated area and the degree of changes was different according to the laser power and reimplantation time (Fig. 5C). However, H&E staining could not precisely identify the thermally damaged area and regeneration of chondrocytes like cell viability test. Cell viability test showed much superior result in determining cellular change by thermal damage. The extent of thermal damage demonstrated by the cell viability test was found to be greater than that demonstrated by conventional histologic examination.
Laser mediated cartilage reshaping (thermochondroplasty) is a non-ablative, low-intensity interaction where heat accelerates stress relaxation in deformed cartilage specimens resulting in shape change. Cartilage can be reshaped when heated to approximately 60℃-75℃[9]. Several different types of lasers have been employed (CO2, Er: Glass, Holmium etc.) with similar results for the treatment of nasal septal deviations [1,10] and protruding ears [3].
LCR relies on heat generation to induce structural changes in the cartilage matrix. Heat causes physical changes in tissue that may not only result in reshaping, but may also injure chondrocytes [1,6,7,9,11-16]. Identifying the appropriate laser parameters is most important in order for LCR to be accepted clinically, without causing thermal injury. The present work focused on determining how viability and regeneration of cartilage varies by laser parameters using a 1,460 nm diode laser.
The wavelength of laser is important for better outcome after LCR. Since the longer wavelength produce deeper penetration, there are chances that the cartilage becomes perforated after laser irradiation if the wavelength is too long. Several wavelengths of lasers have been evaluated in various studies: Nd:YAG laser (λ=1,320 nm); Ho:YAG laser (λ=2,100 nm), and CO2 laser (λ=1,060 nm) [16]. High absorption of CO2 laser causes shallow penetration, while low absorption of Ho:YAG and Nd:YAG laser causes deep penetration with a risk of septal perforation. This study used a 1,460 nm diode laser, of which the wavelength lies between that of CO2 and Ho:YAG that the penetration depth lies in the middle.
We showed that the depth and width of immediate thermal damage increased in proportion with increase in laser power delivered, with significant shape changes both in human septal cartilage and rabbit auricular cartilage. Choi et al. [15] showed similar results with our study in that the extent of nonviable chondrocytes in human septal cartilage increased with increase in power and exposure time after 1,450 nm diode laser irradiation.
Rabbit auricular cartilage is usually thinner and is composed of elastic cartilage as compared to the human nasal septal cartilage, which is relatively thicker and composed of hyaline cartilage. In spite of those differences, we used auricular cartilage for the following reasons; it can be harvested easily, the size of cartilage is large enough for experiments, the auricle has a good curvature and perichondrium can be preserved after harvest for reimplantation of cartilage.
One interesting finding during harvest of reimplanted auricular cartilage was that the angle of curvature of the auricular cartilage decreased as compared with that made initially at the time of implantation, although we did not measure the curvature angle. The physical property of subperichondrial pocket and the regeneration process of damaged cartilage might influence this phenomenon. An in vivo study using rabbit ear by Mordon et al. [16] showed similar observations that each ear had a tendency to recover its initial shape, although all rabbit ears underwent alteration in their shape and retained it for at least 6 weeks.
In the present study, the damaged chondrocytes regenerated following reimplantation into the subperichondrial pocket. Since cartilage is an avascular tissue, it undergoes a wound healing and tissue repair process different from that of most other soft tissues. The regeneration process of the cartilage is based on an intact perichondrium. Perichondrium is rich in stem cells, is highly vascularized, and has chondrogenic potential. The inner perichondrial layer provides the source of new cartilage, and the outer perichondrial layer provides a fibrous overgrowth when there is a trauma of the cartilage [17,18]. Hence, preservation of intact perichondrium is essential for regeneration of damaged cartilage. However, damaged chondrocytes in 1 W treatment group could not regenerate, even though the perichondria were preserved. The maximum temperature can be reached up to 170℃ in 1 W laser irradiation whereas it can be reached up to 65℃-75℃ in 0.3 W and 0.5 W laser irradiation. The high temperature in 1 W treatment group might impair the regenerative capacity of chondrocytes.
Many techniques can be used to assess chondrocyte viability [19,20]. Gauging acute thermal injury may be best accomplished using a live/dead viability assay system combined with laser confocal microscopy [21-24]. Mainil-Varlet et al. [24] reported that the live/dead assay identified a zone of thermal damage that twice exceeded the damage seen by light microscopy, which is consistent with our data. Our study also showed that H&E staining ambiguously showed the characteristics of damaged and regenerated chondrocytes and that live/dead assay revealed the extent of laser damage more accurately than conventional H&E staining. Several other studies have also used live/dead viability assay to demonstrate that thermal damage coexisted with clinically significant shape change in animal septal cartilage [6-8].
In this study, cartilage was harvested and irradiated ex vivo that it is difficult to apply our data in clinical setting such as septoplasty, where cartilage is covered with mucosal layer. In clinical situation, laser is usually irradiated directly on skin or mucosal surface that the effect of mucosal layer was not considered. It is difficult to obtain composite septal cartilage grafts, since the excision of the full thickness of the septum causes septal perforation and is a rare procedure clinically [2].
LCR may offer many advantages over the traditional septal operations if some clinical problems are solved. Ovchinnikov et al. [2] described a large clinical trial of septal cartilage reshaping using transmucosal laser irradiation. Their report showed that LCR was found to be reasonably efficient and safe for the correction of deviated septum. LCR can help to improve the septal deformity like high deviation which is hard to correct by traditional method and to manipulate cartilage graft as the shape we want in rhinoplasty. Although other in vivo and clinical studies showed favorable results of LCR, further study is needed to verify the effect of LCR in mucosa-covered cartilage for clinical application of LCR.
In conclusion, the degree of thermal damage on chondrocytes was related to exposure time and power of laser irradiation. Necrosis, not apoptosis, caused cartilage thermal damage in LCR and the damaged chondrocytes could regenerate after reimplantation between perichondria following appropriate laser irradiation. The present study provides valuable information and it can be used to adapt LCR to clinical use.
ACKNOWLEDGMENTS
This research was supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (2012K1A4A3053142).
References
1. Helidonis E, Sobol E, Kavvalos G, Bizakis J, Christodoulou P, Velegrakis G, et al. Laser shaping of composite cartilage grafts. Am J Otolaryngol. 1993; Nov-Dec. 14(6):410–412. PMID: 8285311.

2. Ovchinnikov Y, Sobol E, Svistushkin V, Shekhter A, Bagratashvili V, Sviridov A. Laser septochondrocorrection. Arch Facial Plast Surg. 2002; Jul-Sep. 4(3):180–185. PMID: 12167077.

3. Trelles MA, Mordon SR. Correction of ear malformations by laser-assisted cartilage reshaping (LACR). Lasers Surg Med. 2006; 8. 38(7):659–662. PMID: 16799999.

4. Bourolias C, Hajiioannou J, Sobol E, Velegrakis G, Helidonis E. Epiglottis reshaping using CO2 laser: a minimally invasive technique and its potent applications. Head Face Med. 2008; 7. 4:15. PMID: 18655713.

5. Diaz SH, Nelson JS, Wong BJ. Rate process analysis of thermal damage in cartilage. Phys Med Biol. 2003; 1. 48(1):19–29. PMID: 12564498.
6. Karam AM, Protsenko DE, Li C, Wright R, Liaw LH, Milner TE, et al. Long-term viability and mechanical behavior following laser cartilage reshaping. Arch Facial Plast Surg. 2006; Mar-Apr. 8(2):105–116. PMID: 16549737.

7. Karamzadeh AM, Chang JC, Diaz S, E Milner T, Wong BJ. Long-term in vivo stability of rabbit nasal septal cartilage following laser cartilage reshaping: a pilot investigation. Lasers Surg Med. 2005; 2. 36(2):147–154. PMID: 15704163.

8. Li C, Protsenko DE, Zemek A, Chae YS, Wong B. Analysis of Nd:YAG laser-mediated thermal damage in rabbit nasal septal cartilage. Lasers Surg Med. 2007; 6. 39(5):451–457. PMID: 17565732.

9. Sobol E, Sviridov A, Omel'chenko A, Bagratashvili V, Kitai M, Harding SE, et al. Laser reshaping of cartilage. Biotechnol Genet Eng Rev. 2000; 17:553–578. PMID: 11255681.

10. Velegrakis GA, Papadakis CE, Nikolidakis AA, Prokopakis EP, Volitakis ME, Naoumidi I, et al. In vitro ear cartilage shaping with carbon dioxide laser: an experimental study. Ann Otol Rhinol Laryngol. 2000; 12. 109(12 Pt 1):1162–1166. PMID: 11130831.

11. Gaon MD, Ho KH, Wong BJ. Measurement of the elastic modulus of porcine septal cartilage specimens following Nd: YAG laser treatment. Lasers Med Sci. 2003; 18(3):148–153. PMID: 14505198.

12. Ho KH, Diaz Valdes SH, Protsenko DE, Aguilar G, Wong BJ. Electromechanical reshaping of septal cartilage. Laryngoscope. 2003; 11. 113(11):1916–1921. PMID: 14603047.

13. Protsenko DE, Wong BJ. Engineering of a straighter septum: numerical model of mechanical stress relaxation in laser-heated septal cartilage. Conf Proc IEEE Eng Med Biol Soc. 2007; 2007:5399–5402. PMID: 18003229.

14. Protsenko DE, Zemek A, Wong BJ. Temperature dependent change in equilibrium elastic modulus after thermally induced stress relaxation in porcine septal cartilage. Lasers Surg Med. 2008; 3. 40(3):202–210. PMID: 18366085.

15. Choi IS, Chae YS, Zemek A, Protsenko DE, Wong B. Viability of human septal cartilage after 1.45 microm diode laser irradiation. Lasers Surg Med. 2008; 10. 40(8):562–569. PMID: 18798294.
16. Mordon S, Wang T, Fleurisse L, Creusy C. Laser cartilage reshaping in an in vivo rabbit model using a 1.54 microm Er:Glass laser. Lasers Surg Med. 2004; 34(4):315–322. PMID: 15083492.
17. Duynstee ML, Verwoerd-Verhoef HL, Verwoerd CD, Van Osch GJ. The dual role of perichondrium in cartilage wound healing. Plast Reconstr Surg. 2002; 9. 110(4):1073–1079. PMID: 12198420.

18. Bruns J, Kersten P, Lierse W, Weiss A, Silbermann M. The in vitro influence of different culture conditions on the potential of sheep rib perichondrium to form hyaline-like cartilage: evaluation of gluing materials used for in vivo graft fixation. Virchows Arch. 1994; 424(2):169–175. PMID: 8180779.

19. Wong BJ, Chao KK, Kim HK, Chu EA, Dao X, Gaon M, et al. The porcine and lagomorph septal cartilages: models for tissue engineering and morphologic cartilage research. Am J Rhinol. 2001; Mar-Apr. 15(2):109–116. PMID: 11345149.

20. Rasouli A, Sun CH, Basu R, Wong BJ. Quantitative assessment of chondrocyte viability after laser mediated reshaping: a novel application of flow cytometry. Lasers Surg Med. 2003; 32(1):3–9. PMID: 12516064.

21. Lu Y, Hayashi K, Hecht P, Fanton GS, Thabit G 3rd, Cooley AJ, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000; Jul-Aug. 16(5):527–536. PMID: 10882450.

22. Lu Y, Edwards RB 3rd, Kalscheur VL, Nho S, Cole BJ, Markel MD. Effect of bipolar radiofrequency energy on human articular cartilage: comparison of confocal laser microscopy and light microscopy. Arthroscopy. 2001; 2. 17(2):117–123. PMID: 11172239.
23. Zuger BJ, Ott B, Mainil-Varlet P, Schaffner T, Clemence JF, Weber HP, et al. Laser solder welding of articular cartilage: tensile strength and chondrocyte viability. Lasers Surg Med. 2001; 28(5):427–434. PMID: 11413554.
24. Mainil-Varlet P, Monin D, Weiler C, Grogan S, Schaffner T, Zuger B, et al. Quantification of laser-induced cartilage injury by confocal microscopy in an ex vivo model. J Bone Joint Surg Am. 2001; 4. 83(4):566–571. PMID: 11315786.

Fig. 1
Schematic illustration of experimental protocol. (A) Experimental protocol of human septal cartilage (B) experimental protocol of rabbit auricular cartilage.
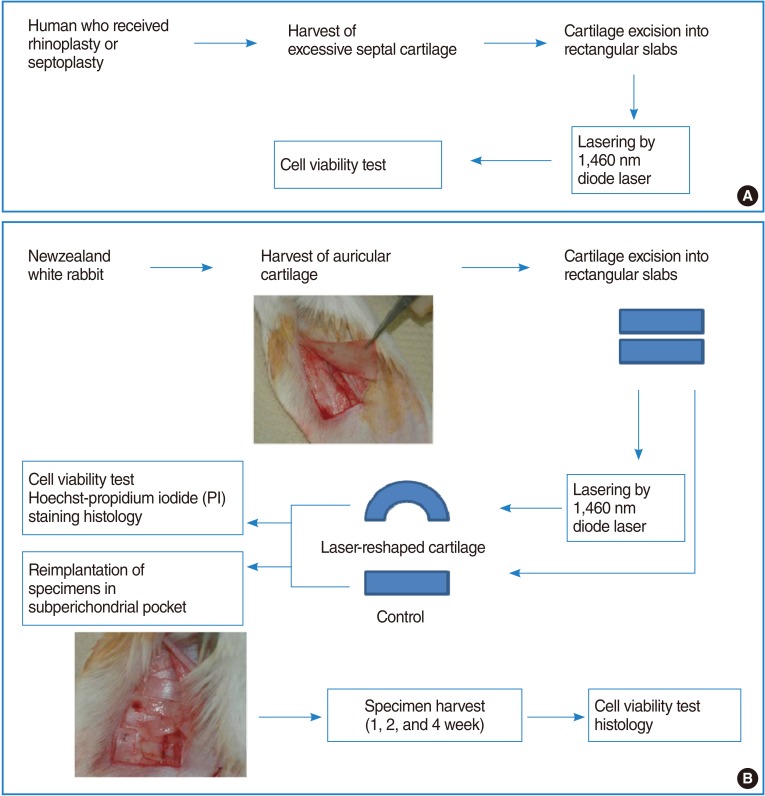
Fig. 2
Shape change of human septal cartilage before (A), immediately after (B) diode laser irradiation. The cartilage was bent by laser irradiation. Reshaped cartilage was recovered into flat shape after re-irradiation by laser to the convex side (C). Red arrow indicates the direction of laser irradiation.
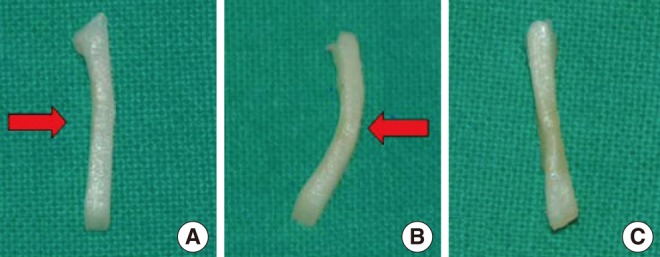
Fig. 3
Confocal images of live/dead assay of the human septal cartilage after (A) spot-pattern laser irradiation and (B) linear pattern laser irradiation. Cartilage was irradiated with different laser power (0.5-2.0 W) and exposure time (5-20 seconds). The green and red fluorescence indicates live and dead cell respectively. The extent of damaged area increased with the increase of the laser power and exposure time irrespective of exposure pattern. Average depth and width of thermal injury following laser cartilage reshaping (human) is plotted in graphs, showing linear increase of damaged depth and width with increase in power and exposure time.
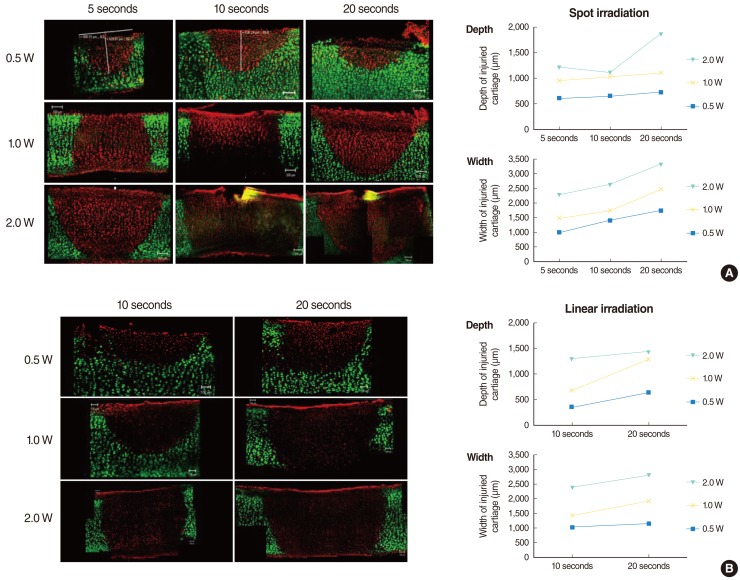
Fig. 4
(A) Confocal images of live/dead assay of the cartilage injury after laser irradiation in rabbit auricular cartilage. Cartilage was irradiated with laser power of 0.3 W, 0.5 W, and 1.0 W for 5 seconds. The extent of damaged area increased with increase in laser power (0.3-1.0 W). (B) Hoechst & PI staining imaging of rabbit auricular cartilage following laser irradiation (laser power, 0.3-1.0 W; exposure time, 5 seconds). Blue and red stained cell indicates live and necrotic cell, respectively. This image shows that thermal injury of chondrocytes resulted in necrosis of chondrocytes rather than apoptosis.
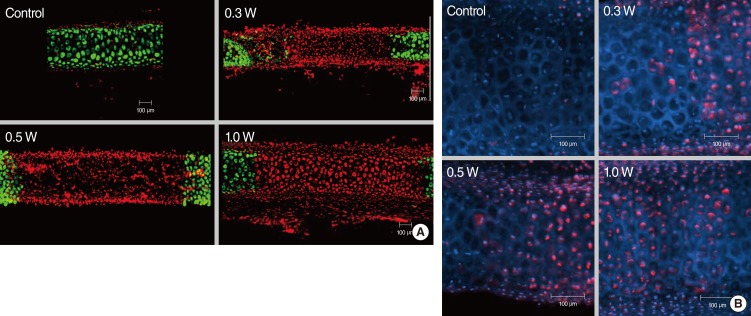
Fig. 5
Regeneration of rabbit cartilage after thermal injury. (A) Confocal images of live/dead assay show that chondrocytes exhibited necrotic changes immediately after laser irradiation, irrespective of laser power. The chondrocytes in 0.3 W and 0.5 W treatment group regenerated completely and those in 0.3 W treatment group showed earlier regeneration. Chondrocytes in 1 W treatment group could not regenerate until 4 weeks after reimplantation. (B) Change of live/dead chondrocyte proportion in irradiated rabbit after reimplantation. (C) Histologic cross sections of laser-irradiated specimens of rabbit cartilage revealed loss of chondrocytes and condensation of nucleus (H&E, ×200).
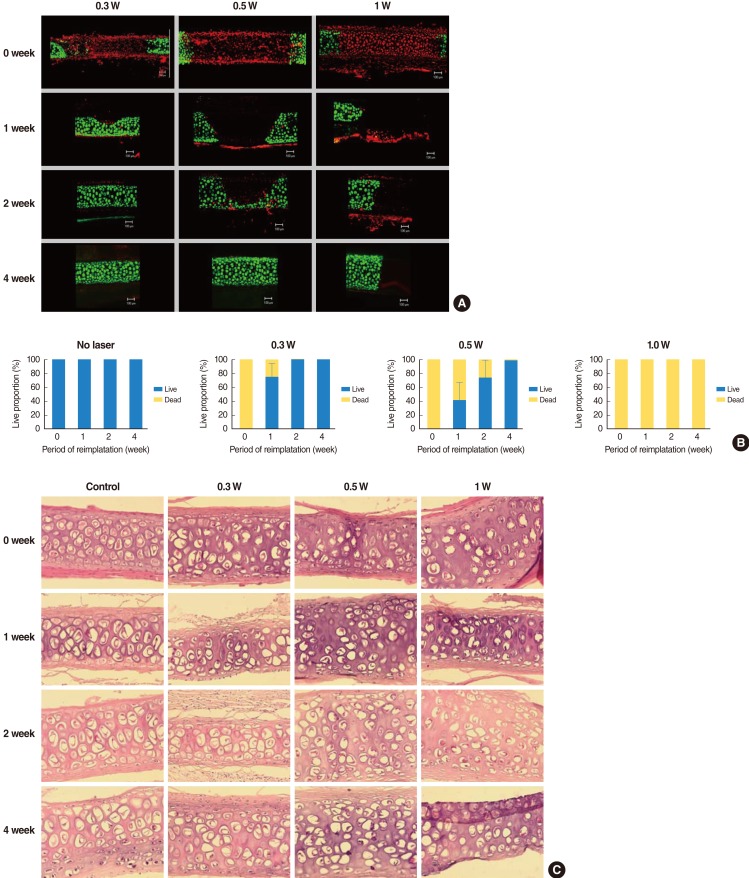
Table 1
Mean depth and width of thermal damage in rabbit auricular cartilage following laser irradiation for 5 seconds (mean±SD)




 Citation
Citation Print
Print


 XML Download
XML Download