Abstract
Objectives
This is to report treatment results of major salivary gland cancer by surgery with or without postoperative radiation therapy (PORT).
Methods
Between March 1995 and January 2006, 94 patients with primary major salivary cancer underwent curative surgical resection at Samsung Medical Center. The parotid gland was the most commonly involved (73, 77.7%), followed by the submandibular and the sublingual. Neck dissection was added in 28 patients, and PORT was individually recommended to those with risk factors. Seventy-five (79.8%) patients received PORT. PORT volume included primary tumor bed and pathologically involved regional lymphatics, and no additional effort was made for elective nodal irradiation. The median total doses were 56.0 Gy to primary site and 58.7 Gy to regional lymphatics.
Results
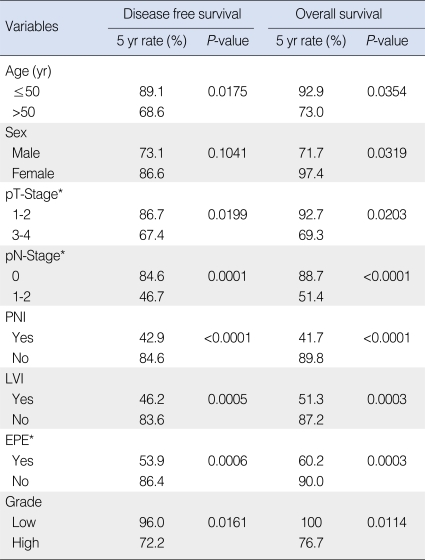
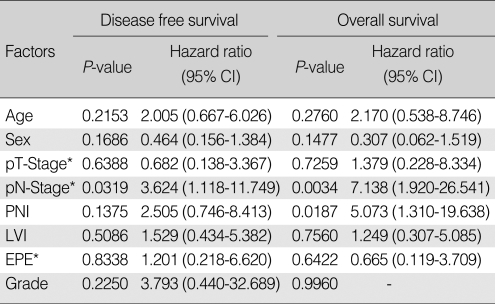
After median follow-up of 49 months, 21 patients had relapsed: 20 in PORT; and one in surgery alone group. As the first site of failure, distant metastasis was the most common (17 patients). Local recurrence occurred in three, and regional relapse in one. The lung was the most common site (10 patients), followed by the bone, and the brain. Five-yr disease free survival (DFS), local control, and overall survival (OS) rates were 74.4% and 94.7%, 96.0% and 100%, and 78.2% and 100% in PORT and surgery alone groups, respectively. On multivariate analysis, DFS was significantly affected by pN+ (hazard ratio [HR], 3.624; P=0.0319), while OS was by pN+ (HR, 7.138; P=0.0034) and perineural invasion (HR, 5.073; P=0.0187).
Conclusion
Based on our experience, the patients with early stage major salivary gland cancer with low risk can be effectively treated by surgery alone, and those who with risk factors can achieve excellent local and regional control by adding PORT. Omitting elective neck irradiation in patients with N0 disease seems a feasible strategy under accurate clinical evaluation. An effort is needed to decrease distant metastasis through further clinical trials.
Malignant tumor of the salivary gland is rather uncommon and accounts for less than 0.3% of all malignancies. The overall incidence in the United States is about 1.2 per 100,000 populations per yr (1). Salivary cancer is heterogeneous with respects to histologic type and biologic behavior. Surgical resection is the mainstay of treatment, and several retrospective studies revealed that the addition of postoperative radiation therapy (RT) in selected patients improved both local control and survival (2-6). The clinical indications of postoperative RT, in general, include pT3-4 disease, positive or close resection margin, bone invasion, perineural invasion (PNI), high-grade histology, and regional lymph node (LN) metastases (3, 7-9). Elective neck irradiation (ENI) is optionally considered for large and high-grade tumors (8-10).
Recent technical improvements have provided us with more accurate diagnosis and optimal treatment planning: computed tomography (CT), magnetic resonance imaging (MRI), and fluorine-18 fluorodeoxyglucose (18F-FDG) whole-body positron emission tomography (PET) help identification of LN metastases with a sensitivity of 80% to 90% and a specificity of 79% to 94% (11); and three-dimensional conformal radiation therapy (3D CRT) and intensity-modulated radiation therapy (IMRT) enable more conformal target coverage with sparing of the normal tissue (12).
The purpose of this study is to report our treatment experience of the patients with major salivary gland cancer, who were treated with surgical resection with or without postoperative RT, and to identify the predictors of outcomes.
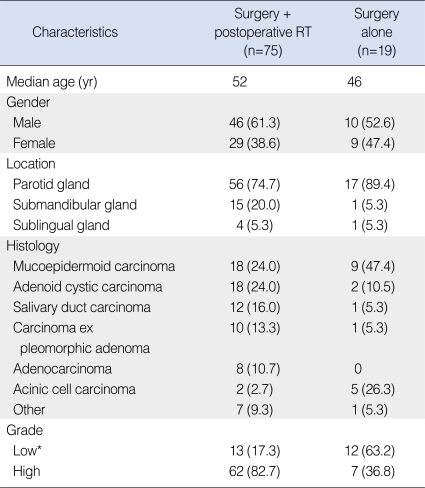
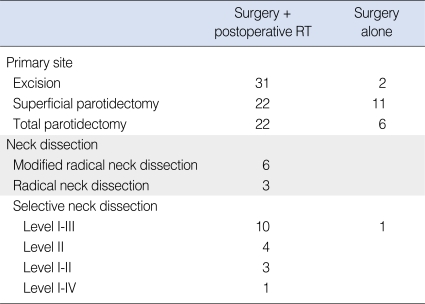
Between March 1995 and January 2006, 94 patients with newly diagnosed major salivary gland carcinoma with no distant metastasis underwent curative surgical resection at Samsung Medical Center. The median age was 50 yr (range, 9 to 79 yr) and there was male preponderance (59.6%, 56 patients). The parotid gland was most commonly involved in 73 patients (77.7%), followed by the submandibular gland in 16 (17.0%) and the sublingual gland in five (5.3%) (Table 1). Surgical procedures included curative resection of the involved gland in all patients, and some form of neck node dissection was added in 28 patients (29.8%) (Table 2). The indication of neck dissection and the decision about type of neck dissection was individualized according to primary tumor site and extent, lymphatic drainage, and clinically involvement of lymph node. At the time of diagnosis, all patients received CT evaluation and some of them received MRI (20 patients) or 18F-FDG PET (21 patients) selectively.
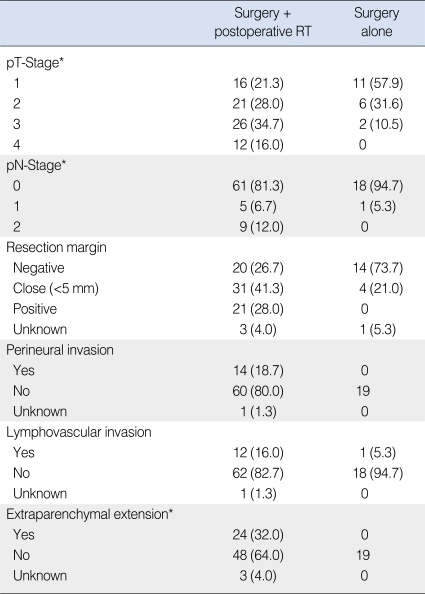
The histologic types are presented in Table 1. Among the 27 patients with mucoepidermoid carcinoma, 19 patients had low-grade tumor and the remaining 8 patients had intermediate or high grade tumor. We considered low-grade mucoepidermoid and acinic cell carcinoma as low-grade tumors, and the other subtypes were considered high-grade. About three fourths of all (73.4%, 69 patients) were with high-grade tumors. The pathologic T-stages according to American Joint Committee on Cancer (AJCC) 6th 2002 are presented in Table 3. Among the 28 patients who underwent neck dissection, 15 (16.0%) had pathologically positive neck node: pN1 in six (6.4%); and pN2 in nine (9.6%). Based on the distribution of characteristics among the patients did or did not receive postoperative RT, those who received postoperative RT had high-grade tumors more frequently (Table 1).
The addition of postoperative RT was considered and individually recommended to the patients with one or more of the following risk factors: high-grade histology; positive or close (<5 mm) resection margin; PNI; lymphovascular invasion (LVI); extraparenchymal extension (EPE); T3-4 disease; and positive regional LN. The criteria of EPE were according to AJCC 6th 2002. The actual numbers of patients who did and did not receive postoperative RT were 75 (79.8%), and 19 (20.2%), and the postoperative RT group had more patients with risk factors (Table 3).
Postoperative RT was delivered with 4- or 6-MV photons generated from linear accelerator. The RT volume was to include the primary tumor bed and pathologically involved regional lymphatics with adequate margins, and no additional effort was made for ENI. CT-based contouring and radiation dose calculation were performed using computerized planning software. The median total doses to the primary site (75 patients) and the regional lymphatics (20 patients) were 56.0 Gy (range, 54 to 70 Gy) and 58.7 Gy (range, 40 to 66 Gy), with a fraction dose of 1.8 Gy (7 patients) or 2.0 Gy (68 patients).
The median follow-up duration, calculated from the date of surgery, was 49 months (range, 6 to 148 months). Complications during or after postoperative RT were scored by the Radiation Therapy Oncology Group (RTOG) criteria. The Kaplan-Meier method was used to estimate the probability of disease-free survival (DFS), local control (LC), and overall survival (OS). The comparisons of survival rates among the groups were done using the log rank test. The Cox proportional hazard regression analysis was used for multivariate analysis. A probability value of less than 0.05 was considered statistically significant.
The most common RT-related toxicities were buccal mucositis on the affected side occurring in 19 patients, dysphagia in 16, and skin reaction in 14. Other minor toxicities included external otitis, dry mouth, and nausea. All the toxicities were either RTOG grade 1 or 2 affecting 53 patients (70.7%), and there was no incidence of grade 3 or higher toxicity.
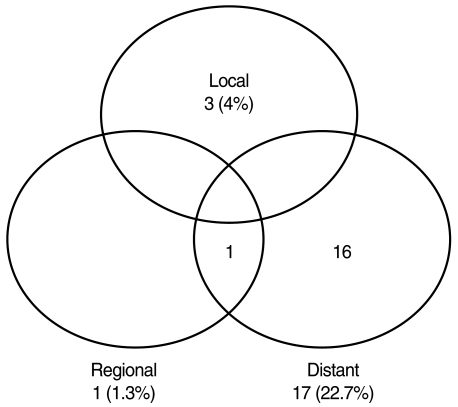
At the time of analysis, 21 patients had relapsed: 20 patients in the postoperative RT group; and one in the surgery alone group. As the first site of failure, distant metastasis was the most common observed in 17 patients, and there were three patients with local recurrence and one with regional relapse (Fig. 1). The sites of distant metastasis were the lung in 10 patients, the bone in three, the brain in two, and other sites in two. Histologic types of 17 patients with distant metastasis were salivary duct carcinoma in six, adenoid cystic carcinoma in six, adenocarcinoma in two, carcinoma ex pleomorphic adenoma in two, and mucoepidermoid carcinoma in one. One patient with simultaneous distant metastasis and regional neck node recurrence was with a few risk factors: histologic type of salivary duct carcinoma; pT3; EPE (+); and positive resection margin. Median time to distant metastasis was 11 months (range, 3 to 34 months). The 5-yr DFS rates were 74.4% and 94.7% in the postoperative RT and the surgery alone groups. All patients who relapsed after postoperative RT had high grade histology, and three patients had local recurrence after 5, 10, and 13 months of surgery, whose histologic types were salivary duct carcinoma in two and adenocarcinoma in one. One patient with regional LN relapse after surgery alone had refused postoperative RT recommended for having pathologically proven LN metastasis. This patient received, after regional recurrence, modified radical neck dissection followed by postoperative RT to the lymphatics, and there has been no evidence of further recurrence until the last follow-up at 9 months after salvage neck dissection. The 5-yr LC rates of the postoperative RT and the surgery alone groups were 96.0% and 100%, and the 5-yr OS rates were 78.2% and 100%.
Table 4 shows results of univariate analysis for factors predictive of DFS and OS. Old age (>50 yr), advanced pathologic T-stage, positive pathologic N-stage, PNI, LVI, EPE, and high grade tumor were significant prognostic factors affecting both DFS and OS. Male sex was a significant factor affecting OS. On multivariate analysis, DFS was significantly affected by positive pathologic N-stage (hazard ratio [HR], 3.624; P=0.0319), while OS was by positive pathologic N-stage (HR, 7.138; P=0.0034) and PNI (HR, 5.073; P=0.0187) (Table 5).
The management of salivary gland cancer is primarily surgical. Early stage major salivary gland cancer with low risk could be effectively cured by surgical resection only. Chen et al. (9) reported treatment results of 207 patients who were treated with surgery alone for major salivary gland cancer. The 10-yr locoregional control of patients with pathologic T1-2 disease was 80% and that of patients without LN metastasis was 77%. LN metastasis (HR, 4.8; P=0.001), high-grade tumor (HR, 4.2; P=0.003), positive surgical margins (HR, 2.6; P=0.03), and T3-4 disease (HR, 2.0; P=0.04) were significant independent predictors of locoregional control. In our study, surgery alone could achieve excellent outcome in patients with low risk, except one patient who refused postoperative RT despite of presence of pathologically positive LN. Except this patient, there was no recurrence or death in the surgery only group.
Patients with advanced T stage, high-grade tumor, positive surgical margins, and LN metastasis usually receive postoperative RT (4, 7, 13-15). In the treatment results of Terhaard et al. (8), postoperative RT improved 10-yr LC significantly compared with surgery alone in patients with T3-4 tumors (84% vs. 18%), close resection margin (95% vs. 55%), incomplete resection (82% vs. 44%), bone invasion (86% vs. 54%), and PNI (88% vs. 60%). LC rates at 5 and 10 yr were 94% and 91%, and with ENI to 40% of patients (a median dose of 50 Gy), postoperative RT also significantly improved regional control in patients with pathological LN involvement (86% vs. 62%). Our study showed 5-yr LC of 96.0% after postoperative RT, which is comparable to the previous reports (4, 6, 8, 13, 14). Furthermore, only one patient experienced regional failure after postoperative RT who had simultaneous distant metastasis. Although we did not administer ENI to clinically or pathologically negative neck, this result is comparable to that of Terhaard et al, whose 10 yr regional control after postoperative RT was 93% for clinically or pathologically N0 tumors (8).
Whether ENI should be applied routinely or not is still a controversial issue in the salivary gland cancer patients with clinically negative neck. ENI should be considered when high risk is suspected for subclinical nodal involvement. Histology, pathologic grade, and tumor size have been known to be the strongest predictors of occult LN metastasis (10, 16-19). Santos et al. (18) classified histological types into three groups according to the risk for neck metastases, and the risks among the high risk group were 85.7% in salivary duct carcinoma, 78.6% in squamous cell carcinoma, 57.1% in high-grade mucoepidermoid carcinoma, 55.6% in undifferentiated carcinoma, and 54.6% in adenocarcinoma. In the study of Bhattacharyya et al., the highest increased odds ratios for nodal metastasis were exhibited in facial nerve involvement (odd ratios [OR], 2.28; P=0.001), tumor grade (OR, 1.99; P<0.001), and squamous cell carcinoma (OR, 2.17; P<0.001) (19). Similar results were reported in the study of Chen et al. (9), and the risks of LN metastases without ENI were 67% in squamous cell carcinoma, 50% in undifferentiated carcinoma, 34% in adenocarcinoma, and 29% in mucoepidermoid carcinoma. There was no neck relapse among the patients with adenoid cystic or acinic cell carcinoma regardless of ENI administration. Although based on rather small number of patients, they suggested that ENI be beneficial in patients with salivary duct carcinoma and carcinoma ex pleomorphic adenoma. 10-yr nodal failure rate among the patients with T3-4 disease was twice higher than that of T1-2 disease (13% vs. 6%, P=0.07), and they concluded that ENI effectively prevented nodal relapses and should be used for selected patients with high risk of regional failure.
Before the decision about whether ENI should be administered or not, accurate clinical evaluation of N-stage is crucial. 18F-FDG PET is known to have significant impact on the management of patients with salivary gland cancers for both the initial staging and the restaging (20, 21). Recently, Jeong et al. (22) reported the diagnostic values from CT and PET/CT scans in patients with high-grade salivary gland cancer. PET/CT predicted neck node metastasis more accurately than using only CT (97.6% vs. 86.0%, P=0.01). Sensitivity and negative predictive value were 100% for cervical LN metastasis. In addition, diagnostic accuracy of PET/CT for predicting the pathologic tumor extent was 91.0%, which was also significantly higher than that of CT alone (70.1%, P<0.001). Based on these, it may be speculated that the use of PET/CT has a major positive impact on the clinical decision-making process for the patients with high-grade salivary gland cancer.
Although excellent local or regional control could be achieved after postoperative RT, the majority of treatment failures were distant metastases. Additional distant metastases can be detected by longer follow-up because distant metastases in salivary gland cancer often occur at over 5 yr and follow-up period was relatively short in current study (23, 24). Improvements in systemic therapy are needed to overcome distant metastasis. Recently, one study regarding trastuzumab therapy for salivary gland carcinoma was published (25). Three patients with recurrence, whose tumors were positive for human epidermal growth factor receptor 2 (HER-2) on fluorescence in situ hybridization, received trastuzumab therapy. One of three patients died after 20 months and the other two were alive at 19 and 36 months, respectively. One of the latter patients has shown disappearance of pulmonary nodules via repeat CT and PET and has remained disease free for 3 yr on trastuzumab. Like this results, HER-2 is a potential target in salivary duct carcinoma. In addition, epidermal growth factor receptor can be a molecular target in adenoid cystic carcinoma, mucoepidermoid carcinoma, and salivary duct carcinoma (26). Further studies of agents targeting these receptors are warranted.
In conclusion, early stage major salivary gland cancer with low risk can be effectively treated by surgical resection only. And patients who had risk factors after surgical resection achieved excellent local and regional control after adding postoperative RT. Under accurate clinical evaluation and appropriate patient selection, omitting ENI in clinically or pathologically N0 disease can be a feasible strategy in patients without risk factors for subclinical nodal involvement. An effort is needed to decrease distant metastasis through further clinical trials.
References
1. United States Cancer Statistics: 1999-2004 incidence and mortality web-based report [Internet]. National Cancer Institute. 2007. cited 2008 Nov 6. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute;Available from: http://www.cdc.gov/cancer/npcr/usc.
2. Theriault C, Fitzpatrick PJ. Malignant parotid tumors: prognostic factors and optimum treatment. Am J Clin Oncol. 1986; 12. 9(6):510–516. PMID: 3788853.

3. Armstrong JG, Harrison LB, Spiro RH, Fass DE, Strong EW, Fuks ZY. Malignant tumors of major salivary gland origin: a matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990; 3. 116(3):290–293. PMID: 2306346.

4. North CA, Lee DJ, Piantadosi S, Zahurak M, Johns ME. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1990; 6. 18(6):1319. 1326. PMID: 2115032.

5. Frankenthaler RA, Luna MA, Lee SS, Ang KK, Byers RM, Guillamondegui OM, et al. Prognostic variables in parotid gland cancer. Arch Otolaryngol Head Neck Surg. 1991; 11. 117(11):1251–1256. PMID: 1747227.

6. Spiro IJ, Wang CC, Montgomery WW. Carcinoma of the parotid gland: analysis of treatment results and patterns of failure after combined surgery and radiation therapy. Cancer. 1993; 5. 01. 71(9):2699–2705. PMID: 8467451.

7. Bell RB, Dierks EJ, Homer L, Potter BE. Management and outcome of patients with malignant salivary gland tumors. J Oral Maxillofac Surg. 2005; 7. 63(7):917–928. PMID: 16003616.

8. Terhaard CH, Lubsen H, Rasch CR, Levendag PC, Kaanders HH, Tjho-Heslinga RE, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005; 1. 01. 61(1):103–111. PMID: 15629600.

9. Chen AM, Granchi PJ, Garcia J, Bucci MK, Fu KK, Eisele DW. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2007; 3. 15. 67(4):982–987. PMID: 17241753.

10. Armstrong JG, Harrison LB, Thaler HT, Friedlander-Klar H, Fass DE, Zelefsky MJ, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992; 2. 01. 69(3):615–619. PMID: 1730113.

11. Adams S, Baum RP, Stuckensen T, Bitter K, Hor G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med. 1998; 9. 25(9):1255–1260. PMID: 9724374.
12. Nutting CM, Rowbottom CG, Cosgrove VP, Henk JM, Dearnaley DP, Robinson MH, et al. Optimisation of radiotherapy for carcinoma of the parotid gland: a comparison of conventional, three-dimensional conformal, and intensity-modulated techniques. Radiother Oncol. 2001; 8. 60(2):163–172. PMID: 11439211.

13. Garden AS, el-Naggar AK, Morrison WH, Callender DL, Ang KK, Peters LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997; 1. 01. 37(1):79–85. PMID: 9054880.

14. Storey MR, Garden AS, Morrison WH, Eicher SA, Schechter NR, Ang KK. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys. 2001; 11. 15. 51(4):952–958. PMID: 11704316.

15. Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005; 6. 15. 103(12):2544–2550. PMID: 15880750.

16. Frankenthaler RA, Byers RM, Luna MA, Callender DL, Wolf P, Goepfert H. Predicting occult lymph node metastasis in parotid cancer. Arch Otolaryngol Head Neck Surg. 1993; 5. 119(5):517–520. PMID: 8484940.

17. Rodriguez-Cuevas S, Labastida S, Baena L, Gallegos F. Risk of nodal metastases from malignant salivary gland tumors related to tumor size and grade of malignancy. Eur Arch Otorhinolaryngol. 1995; 252(3):139–142. PMID: 7662346.

18. Regis De Brito Santos I, Kowalski LP, Cavalcante De Araujo V, Flavia Logullo A, Magrin J. Multivariate analysis of risk factors for neck metastases in surgically treated parotid carcinomas. Arch Otolaryngol Head Neck Surg. 2001; 1. 127(1):56–60. PMID: 11177015.

19. Bhattacharyya N, Fried MP. Nodal metastasis in major salivary gland cancer: predictive factors and effects on survival. Arch Otolaryngol Head Neck Surg. 2002; 8. 128(8):904–908. PMID: 12162768.
20. Otsuka H, Graham MM, Kogame M, Nishitani H. The impact of FDG-PET in the management of patients with salivary gland malignancy. Ann Nucl Med. 2005; 12. 19(8):691–694. PMID: 16444995.

21. Cermik TF, Mavi A, Acikgoz G, Houseni M, Dadparvar S, Alavi A. FDG PET in detecting primary and recurrent malignant salivary gland tumors. Clin Nucl Med. 2007; 4. 32(4):286–291. PMID: 17413575.

22. Jeong HS, Chung MK, Son YI, Choi JY, Kim HJ, Ko YH, et al. Role of 18F-FDG PET/CT in management of high-grade salivary gland malignancies. J Nucl Med. 2007; 8. 48(8):1237–1244. PMID: 17631549.

23. Gurney TA, Eisele DW, Weinberg V, Shin E, Lee N. Adenoid cystic carcinoma of the major salivary glands treated with surgery and radiation. Laryngoscope. 2005; 7. 115(7):1278–1282. PMID: 15995521.

24. Terhaard CH, Lubsen H, Van der Tweel I, Hilgers FJ, Eijkenboom WM, Marres HA, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004; 8. 26(8):681–692. PMID: 15287035.

25. Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007; 10. 29(10):907–912. PMID: 17563907.

26. Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006; 6. 10. 24(17):2673–2678. PMID: 16763282.

Fig. 1
Patterns of failure after postoperative radiotherapy in patients with major salivary gland cancer.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download