Abstract
Objectives
Thioperamide is used as an antagonist to the histamine H3 receptor. During administration of the drug, the trachea may be affected via nasal or oral inhalation. This study was to determine the effects of thioperamide on the trachea of rats in vitro.
Methods
We tested the effectiveness of thioperamide on isolated rat trachea submersed in Kreb's solution in a muscle bath. Changes in tracheal contractility in response to the application of parasympathetic mimetic agents were measured. The following assessments of thioperamide were performed: 1) effect on tracheal smooth muscle resting tension; 2) effect on contraction caused by 10-6 M methacholine as a parasympathetic mimetic; 3) effect of the drug on electrically-induced tracheal smooth muscle contractions.
Results
Thioperamide induced a significant relaxation response at a preparation concentration up to 10-4 M. The drug also inhibited the electrical field stimulation induced spike contraction. However, thioperamide alone had a minimal effect on the basal tension of the trachea at increasing concentrations.
Histamine is known to play a role as a mediator of inflammation, and exerts its effects by activating histamine receptors (HR), of which 4 types are now recognized (H1R, H2R, H3R, and H4R). H3R was firstly postulated by Arrang et al. [1] in 1983 to elucidate the pharmacological properties of the histamine autoreceptor responsible for controlling the release of histamine. It has been reported to exist in the central nervous system, as well as the digestive, cardiovascular, respiratory, genitourinary and immune systems [2]. In the airway, H3R is located in lung, cholinergic ganglia, postganglionic cholinergic nerve, tracheal smooth muscle, bronchial epithelium and nasal submucosal gland [2,3].
Functional studies have revealed several functions of H3R, including: 1) Inhibition of histamine release in histamine nerve terminals. H3R may act to restrict the influx of calcium ions, which is essential for histamine release [1]; 2) Inhibition of histamine synthesis via inhibition of histidine decarboxylase [1]; and 3) Inhibition of the release of other neurotransmitters. H3R stimulation can inhibit the electrically-evoked release of radioactive 5-hydroxytryptamine (5-HT) from rat cerebral cortical slices. In the guinea pig mesenteric artery, perivascular nerve stimulation produces an excitatory junctional potential in vascular smooth muscle cells that can be inhibited by histamine presynaptically. In isolated ileum of the guinea pig, activation of H3R decreased the electrical stimulation-induced contraction [4].
Thioperamide, a selective and competitive H3 antagonist, has been developed. The effect of thioperamide on the trachea is an issue worthy of investigation because during administration of the drug, the trachea may be affected via nasal or oral inhalation. In this study, the effects of thioperamide on isolated tracheal contraction in vitro were investigated by utilizing a unique rat's tracheal model, which could demonstrate the tension change of an intact tracheal ring after addition of the parasympathetic mimetic agents and potential tracheal contraction agents and identify agents that affect tracheal smooth muscle directly.
The chemicals used were of the highest purity available. All chemical reagents were obtained from Sigma (St. Louis, MO, USA). A total of eighteen rats were used in this study, which was approved by the animal experiment review board (LACUC-07-026). Adult rats were anesthetized by intraperitoneal administration of pentobarbital (45 mg/kg), and two pieces of trachea of approximately 5 mm in length were removed from each rat. The tracheal specimens were mounted using two steel plates and submersed in a 30 mL muscle bath at room temperature. The bath was filled with 30 mL Kreb's solution consisting of (mmol/L) NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgSO4 · 7H2O, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; and glucose, 10.0 (Fig. 1). The upper ends of the tracheal strips were attached to a Grass FT-03 force displacement transducer (AstroMed, West Warwick, RI, USA) using a steel plate and a 3-O silk ligature. The other ends of the strips were fixed to a steel plate attached to the bath. A passive tension of 0.3 g was applied to the strips and subsequent changes in tension were recorded continuously using Chart ver. 4.2 (PowerLab, ADInstruments, Colorado Springs, CO, USA).
We tested methacholine as a tracheal contraction drug. Preliminary tests showed that a tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied. Before drug assays were conducted, isolated trachea samples were equilibrated in the bath solution for 15 to 30 minutes, during which continuous aeration with a mixture of 95% O2 and 5% CO2 was applied. Stepwise increases in the amount of drugs used were employed to study the contraction or relaxation responses of the tracheal strips. All drugs were administered by adding a defined volume of stock solution to the tissue bath solution.
Electrical field stimulation (EFS; 5 Hz, 5-ms pulse duration at a voltage of 50 V, trains of stimulation for 5 seconds) was applied to the trachea strips using two wire electrodes placed parallel to the trachea strip and connected to a direct-current stimulator (Grass S44, Grass Technologies, Quincy, MA, USA). An interval of 2 minutes was imposed between each stimulation period to allow recovery from the response. Stimulation was applied contiguously to the trachea at 37℃.
The following effects of thioperamide were assessed: 1) effect on tracheal smooth muscle resting tension: this test was performed to examine the effect of the drug on the simulating condition of resting trachea condition; 2) effect on contraction caused by 10-6 M methacholine: this test was related to the examination of postsynaptic events, such as muscle receptor blockade, enhancement, and second messengers; 3) effect on electrically-induced tracheal smooth muscle contractions: electrical stimulation of this tissue causes the trachea to release parasympathetic transmitter (acetylcholine). If the tested drug interferes with transmitter release, electrical stimulation does not cause contraction. Thus, presynaptic events were seen more easily with this procedure. Accordingly, the treatments in this study included 1) thioperamide alone on the trachea; 2) thioperamide on contracted trachea (by 10-6 M methacholine); and 3) EFS with thioperamide. Each treatment group included 6 tracheal strips for testing.
The concentrations of drugs are expressed as concentrations present in the 30 mL bath solution. An untreated strip served as the control group in each experiment to test the effect of thioperamide on tracheal resting tension or on electrically-induced contraction. In the other experiments with 10-6 M methacholine, the contraction degree of the strip after methacholine addition served as the control value. Compared to the control value, the percentage values were presented as the mean value and standard deviations (SDs) normalized to the control.
Data of the basal tension and methacholine experiment was collected by the mean of tension between two different concentration agent was added. In the EFS experiment, data was the mean of the EFS peak between two different concentration agents was added. Data are presented as mean±SD and statistical significance was tested using a one-way ANOVA; P-values less than 0.01 were considered significant.
The degree of contraction or relaxation of the tracheal strips was estimated from the tension applied to the transducer. The contractile responses of the trachea induced by the parasympathetic agent, methacholine, were easily detected and the tissue remained in a contracted state until the drug was rinsed from the tissue (Fig. 2). The graph variation between each test might be mildly different, but all had the same tendency of results.
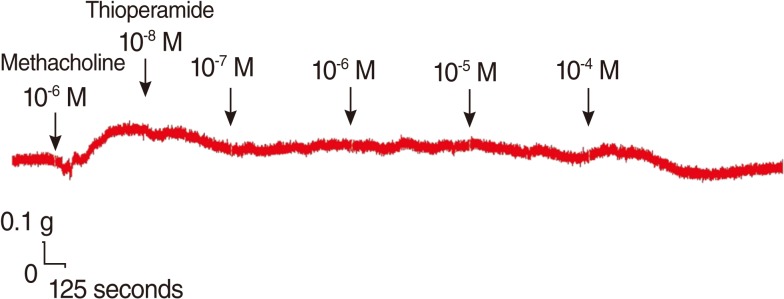
Addition of the H3 antagonist, thioperamide, had a minimal effect on the basal tension (data not shown) but resulted in relaxation of the trachea when introduced after the addition of a constricting agent such as 10-6 M methacholine (Figs. 3, 4). Low doses of thioperamide resulted in a slight decrease in tracheal contraction and higher doses relaxed the trachea much more quickly. The tension was 97.2±2.5%, 95.3±1.6%, 92.0±3.0% and 88.4±3.9% of the control values at 10-8, 10-7, 10-6, and 10-5 M thioperamide, respectively, while at 10-4 M thioperamide, the tension was decreased to 36.7±11.6% (Fig. 3). The inhibition of contraction was statically significant at 10-4 M as compared with that of the control (P<0.01).
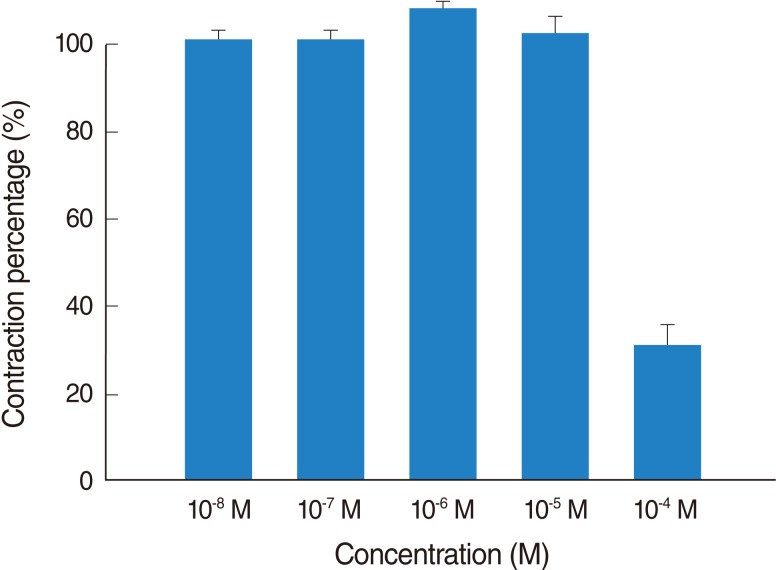
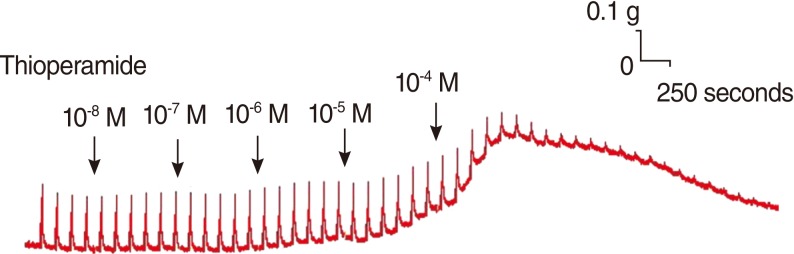
Thioperamide also influenced the spike contraction induced by EFS as well as the basal tension. Although low doses of this drug resulted in little change in the spike contraction, a higher dose of up to 10-4 M prominently lowered the peak tension of the control value. The peak tension of the tracheal strip evoked by EFS upon the addition of 10-6, 10-5 and 10-4 M thioperamide was 106.9±1.5%, 101.5±0.6%, and 30.8±7.5% of the control value, respectively. Following 10-4 M thioperamide addition, the peak tension value of the tracheal strip was significantly lower than that of the control group (P<0.01) (Figs. 5, 6).
Based on the previous techniques [5,6], we developed a modified system that requires only a few millimeters of intact ring of trachea to be excised, and have successfully applied this technique to study the response of the trachea to drugs [7,8]. Although in vitro assays for monitoring tracheal responses to drugs have been developed by other groups [5,6,9-12], our method provides distinct advantages. In such assays, a tracheal mucosa strip measuring 8 mm×20 mm is required to be attached to an isometric transducer and suspended in a tissue bath containing 30 mL of Kreb's solution for the experiment. As a rat tracheal strip of only millimeters in length is required in our experiment, sufficient quantities of trachea are easier to obtain for tests. In addition, a previous in vitro study has demonstrated different degrees of contraction between tracheas with and without an intact tracheal ring [13]. In order to simulate the tracheal response to drugs in vivo, study upon an intact tracheal ring is supposed to be more representative of the physiological situation than previous tests using tracheal smooth muscle alone [5,9,10].
The results of our experiments should be interpreted within the context of the test materials used. The mechanisms of the device were addressed in our previous study [7]. Although it is difficult to determine which tissue component of the trachea was responsible for drug-induced contraction, the nature of the specific tissue and its response to specific drugs provide some indication. First, the tracheal strips used in our study were crude preparations that contained cartilage and tracheal smooth muscle. The smooth muscle of the trachea appeared to be the main tissue component responsible for contraction, as the other components (epithelium, glands, connective tissue, nerves, and cartilage) did not contract to a significant extent. Because the method involves cross contraction, changes in tension were caused by radial contraction of the tracheal ring. Responses to drugs and electrical stimulation have been verified for similar preparations [6,9,10]. However, the contractile response observed was probably an aggregate of the responses of various types of muscle tissue.
Second, the isolated tracheal preparations used in our experiments were excised from rats without damaging the endothelium or smooth muscle. It is therefore reasonable to assume that the tracheal responses to the test agents in our study were comparable with those observed after the application of a spray to the trachea during an asthma attack. As a result of difficulty in obtaining human tissue for similar studies, the effect of this drug on isolated human tracheal smooth muscle still requires further investigation. In the in vivo situation, the response might be much more complicated than that in the in vitro situation.
Histamine, (2-[4-imodazole]-ethylamine), is formed from the amino acid histidine by histidine decarboxylase. There are 3 well-described sources of histamine in humans: mast cells and basophils, gastric enterochromaffin-like cells, and histaminergic nerves in the brain. Histamine is known to play a role as a mediator of inflammation. It is now understood that histamine exerts its effects by activating HR, of which 4 types are now recognized (H1R, H2R, H3R, and H4R). All of these receptors belong to the G protein-coupled receptor family. H1R mediates a series of intracellular events most characterized by changes in free cytosolic calcium levels, whereas H2R mediates intracellular events most characterized by elevations in cyclic adenosine monophosphate (cAMP). The newer family members, H3R and H4R, operate by a variation of both cytosolic free calcium and cAMP [14]. HRs were initially subdivided by Ash and Schild [15] in 1966. These researchers introduced the term H1 receptor to describe the class of HR that is sensitive to inhibition by promethazine and mepyramine, now regarded as H1 receptor antagonists. The classic H1-mediated responses include contraction of many visceral smooth muscles including guinea pig trachea, uterus, and the longitudinal smooth muscle of the ileum. However, acid secretion from the gastric mucosa is resistant to H1R antagonists such as mepyramine. In 1972, Black et al. [16] developed a selective antagonist, burimamide, of the acid-secretion response, which was defined as an H2 receptor.
The third HR (H3) was postulated by Arrang et al. [1] in 1983 to explain the atypical pharmacological properties of the histamine autoreceptor responsible for controlling the release of histamine from rat cerebral cortical slices. In 1987, the pharmacological properties of two potent and selective H3 ligands were reported [17]. H3 receptors now have been recognized to distribute over the central and peripheral tissues with different functions [2,3]. In the airway, H3 receptors may modulate the neurotransmitter release of cholinergic ganglia and postganglionic cholinergic nerves. They also present in the tracheal smooth muscle and bronchial epithelium to induce a relaxation of precontracted trachea [2]. Furthermore, H3 receptors are believed to modulate vascular contractile responses by the inhibition of noradrenaline release from sympathetic nerve terminals in the nasal mucosa, and therefore contribute to the increased mucus secretion of allergic rhinitis [3].
The contracting agent in this test, methacholine, is a synthetic choline ester that acts as a non-selective cholinergic agonist and is commonly used for research purposes. It is worthy of note that the drug-induced relaxation of tissue was dependent on prior partial contraction of the smooth muscle in response to methacholine. It should thus be possible to assay the effects of common drugs and agents supposedly responsible for relieving asthma. Thioperamide, known as a second-generation H3 antagonist [11], has not only been studied in the treatment of central nervous system disorder [18,19], but also in the improvement of symptoms of allergic reaction [20]. Combined with H1 antagonist, thioperamide administration of 1.0-10 mg/kg could be applied for the treatment of allergic diseases of the upper airway [21].
According to the results in methacholine challenge tests, the H3 antagonist, thioperamide was proved to possess the ability to prominently relax the isolated trachea at 10-4 M. This phenomenon indicated a direct anticholinergic effect of thioperamide. Despite the distribution of H3 receptors on smooth muscle [22], their relaxing effects on trachea were not activated due to the absence of histamine in methacholine tests. Hence, it appeared an unknown mechanism for thioperamide to relax the trachea which had been treated with exogenous non-selective cholinergic agonist, methacholine. The interactions with cholinergic or other uncertain receptors were presumably responsible for the anticholinergic effect of thioperamide. Further biomolecular investigation is necessary to clarify this experimental result. Despite the mechanism by which the H3 antagonist affects the trachea smooth muscle remaining unclear, the drug may represent another potential therapy for asthma attacks.
EFS is a common experimental tool that activates the nerve terminals within the tissue and induces the release of endogenous neurotransmitters, followed by triggering the smooth muscle to contract. EFS-induced contraction of canine nasal mucosa disappears after ipsilateral cervical sympathetic ganglionectomy [13], and EFS-induced spike contraction of nasal mucosa was demonstrated by stimulation of sympathetic innervation [13]. In this study, EFS-induced spike contraction of the tracheal smooth muscle was elicited by stimulation of parasympathetic innervation. Therefore, the EFS-induced contraction of the trachea decreased as the concentration of thioperamide increased. This suggests that thioperamide could antagonize not only the effect of H3 agonist, α-MeHA [23], but also parasympathetic innervation in tracheal smooth muscle contraction. The mechanisms of H3 receptors modulating the cholinergic neurotransmitter have been proposed [23], but the physiological influence of H3 receptors on the airway is still uncertain. Our observations in this study were very interesting, and further study is required to clarify these phenomena.
In conclusion, we used this test system employing a short intact ring of trachea to study the effects of thioperamide on tracheal contraction. The study indicated that high concentrations of thioperamide might not only antagonize the effect of cholinergic receptors but also block parasympathetic function of the trachea.
ACKNOWLEDGMENTS
This work was supported in part by the Tri-Service General Hospital (TSGH C97-30) and the National Science Council, R.O.C. (NSC grant 99-2314-B-016-016).
References
1. Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983; 4. 302(5911):832–837. PMID: 6188956.

2. Bertaccini G, Coruzzi G, Poli E. Leurs R, Timmerman H, editors. Functional role of histamine H3 receptors in peripheral tissues. The histamine H3 receptor: a target for new drugs. 1998. Amsterdam: Elsevier;p. 59–111.

3. Suzuki S, Takeuchi K, Majima Y. Localization and function of histamine H3 receptor in the nasal mucosa. Clin Exp Allergy. 2008; 9. 38(9):1476–1482. PMID: 18564330.
4. Valentine AF, Rizzo CA, Rivelli MA, Hey JA. Pharmacological characterization of histamine H3 receptors in human saphenous vein and guinea pig ileum. Eur J Pharmacol. 1999; 1. 366(1):73–78. PMID: 10064154.

5. Bratton DL, Tanaka DT, Grunstein MM. Effects of temperature on cholinergic contractility of rabbit airway muscle. J Appl Physiol. 1987; 11. 63(5):1933–1941. PMID: 3320012.

6. Gonzalez O, Santacana GE. Effect of low temperature on tracheal smooth muscle contractile and relaxing responses evoked by electrical field stimulation. P R Health Sci J. 2001; 9. 20(3):237–244. PMID: 11776725.
7. Wang HW, Wang YT, Wu CC. A modified in vitro method for studying tracheal smooth muscle response to drugs. J Med Sci. 2007; 27(5):203–206.
8. Kao CH, Chu YH, Wang HW. Effects of cetirizine on isolated rat's tracheal smooth muscle. Eur Arch Otorhinolaryngol. 2009; 5. 266(5):753–757. PMID: 18941763.

9. Yau KI, Ko FN, Chien CH. Effects of prokinetic agents on contractile responses to electrical field stimulation of isolated guinea pig trachea. J Formos Med Assoc. 1999; 8. 98(8):567–572. PMID: 10502911.
10. Yau KI, Hwang TL. The nonadrenergic noncholinergic system can modulate the effect of prokinetic agents on contractile response of isolated guinea-pig trachea segments to electrical field stimulation. J Formos Med Assoc. 2002; 10. 101(10):695–699. PMID: 12517043.
11. Schwartz JC, Arrang JM, Garbarg M, Pollard H. Plenary lecture. A third histamine receptor subtype: characterisation, localisation and functions of the H3-receptor. Agents Actions. 1990; 4. 30(1-2):13–23. PMID: 1695431.
12. Wang HW, Jackson RT. Do cholinergic neurons directly innervate nasal blood vessels? Rhinology. 1988; 6. 26(2):139–146. PMID: 3175457.
13. James AL, Pare PD, Moreno RH, Hogg JC. Quantitative measurement of smooth muscle shortening in isolated pig trachea. J Appl Physiol. 1987; 10. 63(4):1360–1365. PMID: 3693169.

14. Bakker RA, Leurs R. Seifert R, Wieland T, editors. Constitutively active histamine receptors. G protein-coupled receptors as drug targets: analysis of activation and constitutive activity. 2005. Weinheim: Wiley-VCH;p. 195–222.

15. Ash AS, Schild HO. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966; 8. 27(2):427–439. PMID: 4381779.

16. Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H2-receptors. Nature. 1972; 4. 236(5347):385–390. PMID: 4401751.
17. Arrang JM, Garbarg M, Lancelot JC, Lecomte JM, Pollard H, Robba M, et al. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987; 5. 327(6118):117–123. PMID: 3033516.

18. Passani MB, Lin JS, Hancock A, Crochet S, Blandina P. The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders. Trends Pharmacol Sci. 2004; 12. 25(12):618–625. PMID: 15530639.

19. Witkin JM, Nelson DL. Selective histamine H3 receptor antagonists for treatment of cognitive deficiencies and other disorders of the central nervous system. Pharmacol Ther. 2004; 7. 103(1):1–20. PMID: 15251226.

20. Amaral MM, Alvarez CA, Langellotti C, Jancic C, Salamone G, Geffner J, et al. Thioperamide induces CD4 CD25 Foxp3 regulatory T lymphocytes in the lung mucosa of allergic mice through its action on dendritic cells. J Asthma Allergy. 2011; 4:93–102. PMID: 22034573.
21. Hansel TT, Barnes PJ, editors. New drugs for asthma, allergy and COPD. 2001. Basel: Karger.
22. Cardell LO, Edvinsson L. Characterization of the histamine receptors in the guinea-pig lung: evidence for relaxant histamine H3 receptors in the trachea. Br J Pharmacol. 1994; 2. 111(2):445–454. PMID: 7911715.

23. Ichinose M, Stretton CD, Schwartz JC, Barnes PJ. Histamine H3-receptors inhibit cholinergic neurotransmission in guinea-pig airways. Br J Pharmacol. 1989; 5. 97(1):13–15. PMID: 2541854.

Fig. 1
Schematic diagram and the actual photo of tension measurement in isolated rat tracheal smooth muscle.
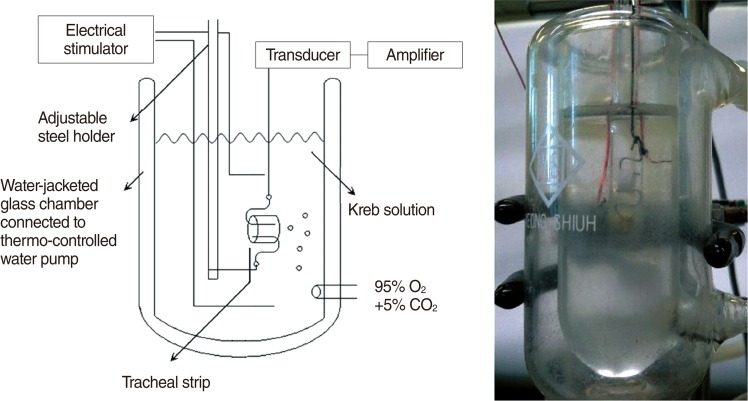
Fig. 2
Tension changes in the rat trachea after the application of methacholine at various concentrations. The basal tension was 0.3 g.
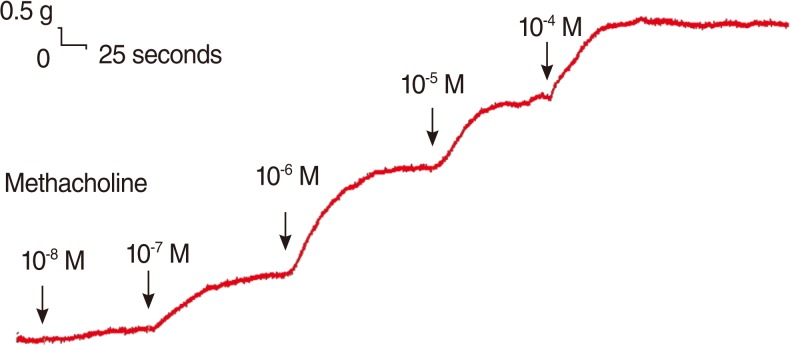
Fig. 4
Effects of thioperamide on 10-6 M methacholine-induced contraction (contraction area was calculated at 100% with no addition of thioperamide) of rat trachea. The inhibition of contraction was statically significant at 10-4 M as compared with that of the control (P< 0.01). The results are represented as the mean±SD (n=6).
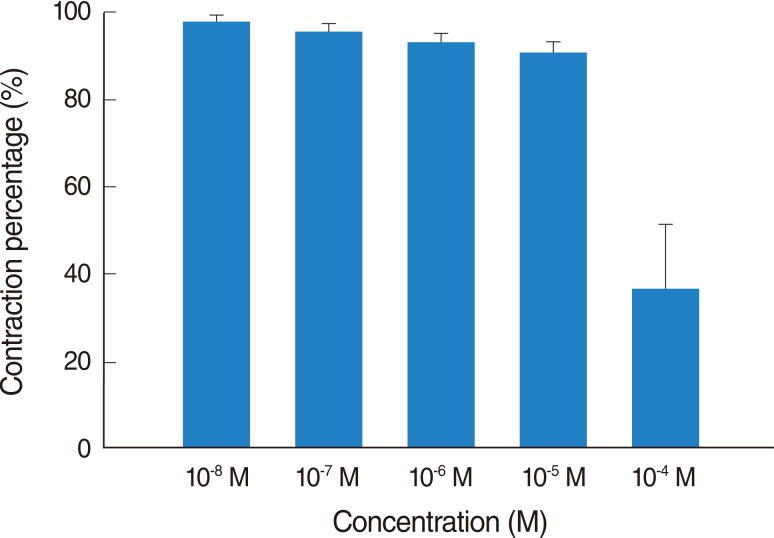
Fig. 6
Effects of thioperamide on electrically-induced tracheal smooth muscle contractions (contraction area was calculated at 100% with no addition of thioperamide). The inhibition of spike contraction was statically significant at 10-4 M as compared with that of the control (P<0.01). The results are represented as the mean±SD (n=6).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download