Abstract
Purpose
To evaluate the visual and anatomical outcomes for neovascular age-related macular degeneration with submacular hemorrhage after intravitreal injections of tenecteplase (TNK), anti-vascular endothelial growth factor (VEGF) and expansile gas.
Methods
This study was a retrospective clinical case series following 25 eyes of 25 patients. All patients received a triple injection using 0.05 mL TNK (50 µg), 0.05 mL anti-VEGF and 0.3 mL of perfluoropropane gas. Retreatment with anti-VEGF was performed as needed. Preoperative and postoperative best-corrected visual acuity and central retinal thickness were analyzed.
Results
The mean logarithm of the minimum angle of resolution of best-corrected visual acuity improved significantly from 1.09 ± 0.77 at baseline to 0.52 ± 0.60 at 12 months (p < 0.001). The mean central retinal thickness also improved significantly from 545 ± 156 at baseline to 266 ± 107 at 12 months (p < 0.001). A visual improvement of 0.3 logarithm of the minimum angle of resolution unit or more was achieved in 15 eyes (60%). During the 12 postoperative months, an average of 4.04 intravitreal anti-VEGF injections was applied.
Submacular hemorrhage (SMH) is a common manifestation of neovascular age-related macular degeneration (nAMD). Left untreated, the prognosis of SMH is poor, especially when the SMH is thick and caused by nAMD [12]. This is a result of a barrier effect that prevents nutrient supply between the retina and the choriocapillaris and toxicity by the iron in the blood [3].
Many treatment options for SMH have been described for the displacement or evacuation of the hemorrhage; these frequently involve the use of intravitreal gas with or without tissue plasminogen activator (tPA) [456]. Pars plana vitrectomy with pneumatic displacement, either with or without tPA may also be considered for massive SMH [7]. Several recent studies have successfully used intravitreal anti-vascular endothelial growth factor (VEGF) agents in combination with pneumatic displacement for treating SMH in patients with nAMD [89].
Tenecteplase (commercial TNK; Metalyse, Boehringer Ingelheim, Sydney, Australia) has greater fibrin specificity, a reduced potential for retinal toxicity and a known ability to penetrate the retina following intravitreal injection compared with tPA [1011121314]. McAllister et al. [14] reported positive results of intravitreal TNK injections for treatment of SMH due to nAMD.
The aim of this study was to evaluate the outcome of a triple injection with intravitreal injection of TNK, anti-VEGF agent, and an expansile gas for the treatment of thick SMH caused by nAMD.
This retrospective study followed the principles of the Declaration of Helsinki and was approved by the institutional review board of the Nune Eye Hospital (no. 2010-NEH-001). For academic use, the Korean Food and Drug Administration approved the intravitreal injection of TNK. The medical records of patients who underwent triple injections from March 2009 to May 2013 at Nune Eye Hospital were retrospectively reviewed.
Inclusion criteria were nAMD complicated by SMH, SMH >1 disk areas, treatment naive, and a <30 day symptom duration with a minimum 12-month follow-up period. Exclusion criteria were other etiologies of SMH, previous intravitreal injection or vitreoretinal surgery and pre-existing macular scarring. Differentiation between typical nAMD and polypoidal choroidal vasculopathy was determined using indocyanine green angiography. Patients who had a branched vascular network with a terminal polypoidal vascular lesion were classified as polypoidal choroidal vasculopathy.
All patients were examined before the treatment, 1 to 7 days after surgery, and at 1 month intervals. Each patient underwent a complete ocular examination including slitlamp examination, best-corrected visual acuity (BCVA), intraocular pressure measurement, fundus photography and spectral-domain optical coherence tomography. Preand postoperative fluorescein angiography and indocyanine green angiography were performed.
Medical records were reviewed for the following data: age, gender, systemic disease, duration of hemorrhage, BCVA with Snellen charts and mean change from baseline in central retinal thickness (CRT) with spectral domain optical coherence tomography (Heidelberg Spectralis; Heidelberg Engineering, Heidelberg, Germany) preoperatively as well as at 1, 3, 6, and 12 months postoperatively. For statistically analyses, BCVA was analyzed on a logarithm of the minimum angle of resolution (logMAR) scale. In the current study, visual acuity of counting fingers and hand motion was converted as 2.0 and 3.0 logMAR, respectively. CRT was measured manually on the optical coherence tomography monitor using the built-in software. CRT was defined as the distance between the internal limiting membrane and Bruch's membrane at the center of the fovea. If the CRT was over 1,000 µm, it was considered as 1,000 µm.
All procedures were performed under local anesthesia and sterile conditions in the operating room. Each substance was injected through the pars plana into the vitreous cavity using a 30-gauge needle. On day 1, TNK (50 µg/0.05 mL) and anti-VEGF (Avastin 1.25 mg/0.05 mL or Lucentis 0.5 mg/0.05 mL; Genentech, San Francisco, CA, USA) were injected into the vitreous cavity. An anterior chamber paracentesis was performed, if deemed necessary. All patients were asked to remain in the supine position for at least 3 hours. Next day, 0.2 mL of the aqueous humor was removed to avoid the elevation of intraocular pressure before the intravitreal gas injection, and 0.3 mL of pure perfluoropropane gas (C3F8) was injected. Patients were instructed to maintain a facedown posture for at least 1 week. After performing the initial triple injection, a retreatment of anti-VEGF for each patient was performed as needed, if persistent or recurrent subretinal or intraretinal fluid was evident or if a new macular hemorrhage developed.
All values are presented as mean ± standard deviation. The Wilcoxon signed rank test was used for comparison of preoperative and postoperative visual acuities and CRT. PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. A p-value of less than 0.05 was considered statistically significant.
A total of 25 treatment-naïve nAMD eyes (25 patients) that underwent the triple injection procedure were included. Patient baseline characteristics are reported in Table 1. The participants consisted of 14 men and 11 women with an average age of 67.6 ± 8.9 years (range, 52 to 82 years). The mean duration of symptoms was 7.2 ± 8.2 days (range, 1 to 30 days) and the mean follow-up duration was 19.6 ± 8.3 months (range, 12 to 41 months). The mean size of the SMH was 7.5 ± 5.0 disc areas (range, 1.5 to 19). Fifteen patients (60%) were treated with bevacizumab, and 10 patients (40%) were treated with ranibizumab. At 12 months postoperative, 23 of 25 eyes had received a mean of 4.04 (range, 1 to 11) postoperative intravitreal injections of bevacizumab or ranibizumab. A history of hypertension was identified in nine patients (36%), diabetic mellitus in five patients (20%), and kidney transplantation in one patient (4%). Five patients (20%) were treated with systemic anticoagulation. Typical nAMD was identified in 19 eyes (76%), and polypoidal choroidal vasculopathy was classified in six eyes (24%).
The mean values of BCVA, expressed in logMAR, and CRT at each visit are reported in Table 2. Both BCVA and CRT significantly improved during the follow-up visits. BCVA improved from preoperative 1.09 ± 0.77 (range, 3.0 to 0.2) to 0.78 ± 0.61 (range, 2 to 0.1) at 1 month (p = 0.009), 0.60 ± 0.54 (range, 2 to 0) at 3 months (p < 0.001), 0.54 ± 0.56 (range, 2 to 0) at 6 months (p < 0.001) and 0.52 ± 0.60 (range, 2 to 0) at 12 months (p < 0.001). Mean CRT improved from preoperative 562 ± 183 µm (range, 197 to 775) to 244 ± 85 µm (range, 118 to 417) at 1 month (p < 0.001), 215 ± 58 (range, 111 to 308) at 3 months (p < 0.001), 250 ± 119 (range, 139 to 659) at 6 months (p < 0.001), and 266 ± 107 (range, 147 to 544) at 12 months (p < 0.001). A visual improvement of 0.3 logMAR unit or more was achieved in 15 eyes (60%) (Fig. 1A-1D) and only one eye showed worsened BCVA at 12 months.
Treatment was well tolerated and there were no cases of infectious endophthalmitis. In one eye, a vitreous hemorrhage developed after the first treatment, and a retinal pigment epithelium tear occurred in one eye during the follow-up period. Other than those two cases no severe ocular or systemic adverse events occurred.
Unless treated early, SMH associated with choroidal neovascularization (CNV) generally cause sudden visual loss and poor visual prognosis [12]. The reduced visual outcome is attributed to the retinal toxicity of subretinal blood, which includes limited diffusion of nutrients and oxygen, shearing off photoreceptor outer segments due to clot contraction and release of toxic materials, such as hemoglobin-derived iron [3]. This is precisely why many investigators have supported early evacuation of SMH to minimize these damaging effects.
Heriot presented the benefits of a minimally invasive procedure to enzymatically liquefy the submacular blood with tPA and displace it with gas [15], and many studies have since shown good results with this procedure [16]. However, the commercially available 50 <g of tPA was reported to be toxic in rabbit, and it caused reduced electroretinograph amplitudes and diffuse pigmentary changes in human retinas [17]. Moreover, several recent reports have shown that pneumatic displacement therapy for SMH was equally effective without regard to the use of tPA [5618].
A new third-generation thrombolytic agent (tenecteplase) is a variant of tPA that has been produced by recombinant DNA technology, having undergone multiple point mutations [13]. This new thrombolytic agent had been developed to avoid some of the limitations of tPA [111213]. With a longer half-life and greater fibrin specificity, TNK is potentially more efficacious in thrombus dissolution [11]. Another advantage of TNK is that the vehicle contains much less L-arginine (less than a third of that of tPA) which is thought to be the cause of the toxicity to the outer retina and RPE. In a previous animal study, subretinal TNK injection showed no toxicity to the outer retina and earlier we reported a case study in which subretinal administration of TNK did not result in serious retinal toxicity in human [19]. Recently, intravitreal TNK injection was reported to yield favorable effects in the management of SMH [14].
Visual acuity often improves after successful displacement of SMH by tPA and gas but frequently deteriorates because of progression of the underlying CNV. To overcome this limitation intravitreal injection of anti-VEGF agents, a proven treatment method for CNV related to nAMD, are being used. The risk of SMH recurrence may be reduced by early CNV regression induced by the simultaneous application of anti-VEGF. Several retrospective studies have successfully used intravitreal anti-VEGF agents in combination with pneumatic displacement for treating SMH in patients with nAMD [89]. Positive results for intravitreal anti-VEGF monotherapy have also been reported to be successful for treating patients with SMH related to nAMD [2021].
The therapeutic principle of triple injection is based on three synergistic effects: the non-toxic and highly penetrating ability of TNK to lyse the clot, the mechanical displacement of submacular liquefied blood through the gas bubble, and the treatment of the underlying CNV with anti-VEGF agent. In the present study, 25-patient case series demonstrates that combined treatment of SMH with intravitreal TNK, anti-VEGF agent and gas is both safe and effective in improving visual acuity from 1.09 ± 0.77 log-MAR to 0.52 ± 0.60 logMAR and in reducing CRT from 545 ± 156 to 266 ± 107 µm at 12 months. Although the results cannot be directly compared with other studies, our results are comparable to those of other previous studies where they used combined injections of anti-VEGF and gas [22] or triple injection of tPA, bevacizumab and gas [9].
Our study has several limitations. First, this is the retrospective study. Second, the number of patients was relatively small and only short-term postoperative evaluations were conducted. Third, our results are limited by the lack of a control group. However, this case series is meaningful as the first clinical report of combination injection in humans, with satisfactory results.
In conclusion, pneumatic displacement with intravitreal TNK and anti-VEGF agent is a safe and effective treatment for nAMD complicated by SMH. Large, long-term, prospective, comparative clinical trials are needed to evaluate the efficacy and safety of this procedure.
Figures and Tables
Fig. 1
Preoperative and 12 months postoperative fundus photographs and optical coherence tomography images of patient 4 (A,B) and patient 12 (C,D).
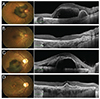
Table 1
Baseline characteristics

Values are presented as number, mean ± standard deviation, or number (%) unless otherwise indicated.
DA = disc areas; nAMD = neovascular age-related macular degeneration; PCV = polypoidal choroidal vasculopathy; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; CRT = central retinal thickness; anti-VEGF = anti-vascular endothelial growth factor.
References
1. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996; 16:183–189.
2. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–37.
3. Hochman MA, Seery CM, Zarbin MA. Pathophysiology and management of subretinal hemorrhage. Surv Ophthalmol. 1997; 42:195–213.
4. Schulze SD, Hesse L. Tissue plasminogen activator plus gas injection in patients with subretinal hemorrhage caused by age-related macular degeneration: predictive variables for visual outcome. Graefes Arch Clin Exp Ophthalmol. 2002; 240:717–720.
5. Cakir M, Cekic O, Yilmaz OF. Pneumatic displacement of acute submacular hemorrhage with and without the use of tissue plasminogen activator. Eur J Ophthalmol. 2010; 20:565–571.
6. Mizutani T, Yasukawa T, Ito Y, et al. Pneumatic displacement of submacular hemorrhage with or without tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1153–1157.
7. Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings. SST report no. 13. Ophthalmology. 2004; 111:1993–2006.
8. Hohn F, Mirshahi A, Hattenbach LO. Combined intravitreal injection of bevacizumab and SF6 gas for treatment of submacular hemorrhage secondary to age-related macular degeneration. Ophthalmologe. 2010; 107:328–332.
9. Meyer CH, Scholl HP, Eter N, et al. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008; 86:490–494.
10. Kwan AS, Vijayasekaran S, McAllister IL, et al. A study of retinal penetration of intravitreal tenecteplase in pigs. Invest Ophthalmol Vis Sci. 2006; 47:2662–2667.
11. Rowley SA, Vijayasekaran S, Yu PK, et al. Retinal toxicity of intravitreal tenecteplase in the rabbit. Br J Ophthalmol. 2004; 88:573–578.
12. McAllister IL, Vijayasekaran S, Khong CH, Yu DY. Investigation of the safety of tenecteplase to the outer retina. Clin Experiment Ophthalmol. 2006; 34:787–793.
13. Davydov L, Cheng JW. Tenecteplase: a review. Clin Ther. 2001; 23:982–997.
14. McAllister IL, Chen SD, Patel JI, et al. Management of submacular haemorrhage in age-related macular degeneration with intravitreal tenecteplase. Br J Ophthalmol. 2010; 94:260–261.
15. Heriot W, editor. Intravitreal gas and tPA: an outpatient procedure for subretinal haemorrhage. Proceedings of Vail Vitrectomy Meeting. Vail: American Society of Retina Specialists;1996.
16. Chen CY, Hooper C, Chiu D, et al. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007; 27:321–328.
17. Chen SN, Yang TC, Ho CL, et al. Retinal toxicity of intravitreal tissue plasminogen activator: case report and literature review. Ophthalmology. 2003; 110:704–708.
18. Fujikawa M, Sawada O, Miyake T, et al. Comparison of pneumatic displacement for submacular hemorrhages with gas alone and gas plus tissue plasminogen activator. Retina. 2013; 33:1908–1914.
19. Kwon YH, Lim SJ, Jeung WJ, et al. Subretinal tenecteplase injection in a submacular hemorrhage from polypoidal choroidal vasculopathy: a case report. Retin Cases Brief Rep. 2012; 6:400–405.
20. Shienbaum G, Garcia Filho CA, Flynn HW Jr, et al. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am J Ophthalmol. 2013; 155:1009–1013.
21. Cho HJ, Koh KM, Kim HS, et al. Anti-vascular endothelial growth factor monotherapy in the treatment of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013; 156:524–531.el.
22. Cho HJ, Koh KM, Kim JH, et al. Intravitreal ranibizumab injections with and without pneumatic displacement for treating submacular hemorrhage secondary to neovascular age-related macular degeneration. Retina. 2015; 35:205–212.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download