Abstract
Purpose
To determine the effect of exogenous nitric oxide (NO) on the migration of trabecular meshwork (TM) cells and its association with expression of matrix metalloproteinases (MMPs).
Methods
Primary human TM cells treated with 1 or 10 µM S-nitroso-N-acetyl-penicillamine (SNAP) and examined for changes in adherence. TM cells were seeded onto transwell culture inserts, and changes in their migratory activity were quantified. Reverse transcription polymerase chain reaction was performed to determine the relative changes in mRNA expression of MMPs and tissue inhibitor of metalloproteinases (TIMPs).
Results
Treatment with SNAP did not significantly suppress TM cell adhesion or migration (p > 0.05). Treatment of TM cells with 10 µM SNAP decreased expression of MMP-2 and increased expression of membrane type MMP-1 and TIMP-2. Treatment with interleukin-1α triggered MMP-3 expression but did not exert significant effects on MMP-3 activation in response to SNAP.
Conclusions
These data suggest that NO revealed no significant effect on the migration of TM cells because NO decreased MMP-2 and increased TIMP-2 expression. Although expression of certain MMPs and TIMPs change in response to NO donors, NO may modulate trabecular outflow by changing the cellular production of extracellular matrix without having a significant effect on the migration of TM cells.
The trabecular meshwork (TM) of the eye, composed of cells and matrix, is thought to regulate aqueous humor outflow to control intraocular pressure (IOP) [1]. Among the factors controlling IOP, a pivotal role is ascribed to the TM, a smooth muscle-like tissue with contractile properties in the anterior chamber angle of the eye that regulates aqueous humor outflow [23]. When these signals are received, extracellular matrix (ECM) turnover is adjusted to shift the balance toward higher or lower resistance [456789]. The significance of matrix metalloproteinases (MMPs) in outflow resistance was shown when treatment of anterior segments in perfusion culture with MMPs was found to increase outflow, while specific inhibition of MMP activity decreased outflow facility [10].
The TM, along with a number of other tissues in the eye has an extremely limited replicative capacity in vivo [11]. With aging, the cell population progressively decreases, defects being made up by cell spreading rather than overt re-proliferation [121314]. If a cell enters the cell cycle to replenish the diminishing meshwork cell population, it will loosen its contact with the ECM with adjacent cells and up-regulate appropriate receptors. Thus, the very cells which are about to divide are the very ones most likely to be lost by mobilization and subsequent migration. Despite evidence that nitric oxide (NO) inhibits cell migration and proliferation in several different experimental models, NO elicits opposite effects on different cell types; namely, the inhibition of vascular smooth muscle cell (VSMC) proliferation but also the promotion of endothelial cell (EC) proliferation [15]. Proliferating VSMCs in intact vascular tissues secrete metalloproteinases that proteolyze matrix proteins, thereby allowing them to migrate [1617]. Trachtman et al. [18] have found that NO stimulates the activity of a 72-kDa MMP (gelatinase) in cultured rat mesangial cells, and Murohara et al. [19] have demonstrated that endothelial nitric oxide synthease derived NO facilitates EC migration [20]. If these findings are also true for TM cells, this would constitute a pathway by which NO might promote migration and result in TM cell loss. Despite the beneficial IOP-lowering effect of NO, this may exacerbate glaucoma. Until now, it has been unclear as to whether NO affects the migratory activity of TM cells.
The purpose of this study was to determine whether NO inhibits the migration of cultured TM cells and to evaluate possible involvement of MMPs related to the regulation of TM cell migration.
S-nitroso-N-acetyl-penicillamine (SNAP), 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT),Nω-nitro-L-arginine methyl ester (L-NAME), Griess reagent, and interleukin-1α(IL-1α) were purchased from Sigma-Aldrich (St. Louis, MO, USA). A microchemoattraction chamber (No. 3415; Transwell, Sigma-Aldrich) was purchased from Corning (Acton, MA, USA). Fetal bovine serum (FBS), Dulbecco's modified eagle's medium (DMEM), penicillin/streptomycin, and trypsin were purchased from Gibco (Invitrogen, Carlsbad, CA, USA). Primers for mRNA were obtained from Genet bio (Seoul, Korea). Trizol was purchased from Invitrogen and Taq Green Master Mix was purchased from Promega (Fitchburg, WI, USA). All other reagents and general lab chemicals were purchased from Sigma-Aldrich Chemical.
TM cell cultures were established from enucleated human eye obtained from the eye bank and transported on ice within two hours after exsanguinations as previously described [21]. Briefly, TM tissues were excised by dissecting a continuous strand of tissue between the line of Schwalbe and the scleral spur by teasing away the TM tissue using a curette. The excised TM tissues were placed in a sterile culture dish with DMEM medium containing 15% FBS, 2 mM glutamine, 50 µg/mL gentamicin, and 2.5 mg/mL fungizone and left undisturbed for three to five days in a 37℃ incubator with a 5% CO2 atmosphere. After identifying initial cell growth, the explants were removed and the cultures were maintained with a medium containing 10% FBS. Cultures of three to five passages were used for experiments.
Cultures approaching confluency were trypsinized and inoculated into 12-well culture plates (1×105 cells/well). After allowing attachment, the cells were washed three times with serum-free medium and cultured for 24 hours in medium lacking FBS but supplemented with 0.5% bovine serum albumin. The cells were then cultured for 24 hours in DMEM supplemented with 1% FBS. The NO donor SNAP was added to the medium at concentrations of 0, 1, or 10 µM with or without co-treatment with the NO inhibitor 0.5 mM L-NAME. To induce certain MMPs, 25 ng/mL IL-1α was added to the cultures. Then, the cultures were returned to the incubator and incubated for 24 hours.
Cell survival was determined by a rapid MTT colorimetric assay. The MTT assay is based on the tetrazolium salt MTT that detects living but not dead cells. As signals are generated, the optical density is directly proportional to the number of cells [22]. After adding 100 µL of a MTT stock solution (5 mg MTT/mL phosphated buffered saline) to each well, all the media was removed from the well after a four-hour incubation at 37℃. Then, 0.5 mL of dimethyl sulfoxide (DMSO) was added to each well, and 100 µL of the solution was transferred from each well to a 96-well plate and read on a multi-well scanning spectrophotometer (λ = 570 nm, Fluostar Optima; BMG Labtech, Offenburg, Germany). A minimum 10-minute exposure to DMSO was required to dissolve the MTT formazan crystals after which the absorbance remained constant for 20 minutes. Therefore, in all experiments, spectrophotometric readings were taken 15 minutes after the addition of DMSO.
Nitrite concentrations in the media were measured using the Griess reaction [23]. Briefly, media samples were collected from each well following appropriate treatment and reacted with modified Griess reagent by mixing equal volumes with each other at room temperature for 15 minutes. Optical density was then measured and read on a multi-well scanning spectrophotometer at 540 nm. The nitrite concentration was then determined from a comparison of absorbance with that of a standard solution of sodium nitrite in medium. The background absorbance, measured using the medium alone, was subtracted from all values.
A TM cell adhesion assay was carried out as described previously with minor modifications [2425]. The cells were seeded in the presence or absence of SNAP or L-NAME in two 96-well plates. After a 60-minute incubation, unattached cells were removed by washing three times with phosphated buffered saline. After removing the unattached cells, cells were quantified by the MTT assay as described above. Cell adhesion (%) was expressed as the number of adhered cells (after washing) divided by the number of total cells (before washing).
The migration of TM cells was measured with a transwell migration apparatus as described previously [2627]. Briefly, cells were trypsinized and resuspended at a density of 2×106 cells/mL. Then, the TM cells were added into the upper wells of a transwell chamber (insert pore size, 3.0 µm). The cultures containing media with or without SNAP were incubated for five hours at 37℃ in an atmosphere of 95% air and 5% CO2. At the end of the incubation period, cells were stained with trypan blue, and migrated cells attached to the bottom of the filter were counted under a light microscope. Cell migration (%) was expressed as the number of migrated cells divided by the number of total cells.
Total RNA was extracted with Trizol (Invitrogen). An RNA denaturation mix consisting of isolated RNA, oligo dT primers, and nuclease-free water was denatured. RT-PCR was performed using oligonucleotide primers specific to MMP-2, -3, -9, -14, and tissue inhibitor of metalloproteinase (TIMP)-1 and-2 mRNA (Table 1) [28]. Two tubes were run in parallel with the second tube containing only Platinum Taq polymerase to assure that the source of the RT-PCR product was mRNA. cDNA was synthesized by adding prime RT premix. Taq Green Master Mix and 10 pM each of forward and reverse primers was added to the synthesized cDNA. The amplification reaction was carried out for 30 cycles on a DNA Engine Cycler (Bio-Rad, Hercules, CA, USA). The amplified PCR products were analyzed using Multi-gauge software (Fujifilm, Tokyo, Japan) after electrophoresis. The level of β-actin was used as an internal standard.
The expression of mRNA was scanned, and the relative intensity of bands was determined by densitometry. Every experiment was performed in triplicates, and the data are expressed as mean ± standard error of the mean corresponding to the number of wells analyzed. Experimental differences between the results of control cultures and a single treatment group were evaluated using the Student's t-test. A p-value less than 0.05 was considered statistically significant. The two-sample z-test was used for comparing two proportions.
The NO donor SNAP significantly increased NO in the media in a dose-dependent manner. Nitrite concentration increased significantly from 0.68 µM in the non-exposed control to 6.62 and 13.21 µM with exposure to 1 or 10 µM SNAP, respectively (p = 0.003, 0.001) (Fig. 1).
However, 25 ng/mL IL-1α had no effect on the production of NO compared to the non-exposed control (p = 0.263). Addition of L-NAME to 10 µM SNAP decreased NO production to 7.39 µM.
SNAP did not significantly inhibit the survival of TM cells at a concentration of 1 or 10 µM (p > 0.05) (Fig. 2). When 0.5 mM L-NAME or 25 ng/mL IL-1α was administered simultaneously with SNAP, cell survival was also not significantly affected (p > 0.05). Thus, the results of these experiments show that cell survival and proliferation were not affected by a concentration of 10 µM SNAP.
To test the possibility that NO interferes with cell matrix adhesion, the attachment of TM to the cell matrix was examined in the presence or absence of SNAP. Incubation of TM cells with either SNAP or the inhibitor of NO synthase L-NAME were plated and allowed to attach for five hours. The percent of adherent cells at 1 or 10 µM was 70.1% ± 1.0% and 71.7% ± 0.7%, respectively. Adhered cells in the controls were 68.4% ± 0.5% of the total added cells (Fig. 3).Treatment with SNAP did not result in a significant blunting of TM cell adhesion. Increasing the concentration of SNAP up to 10 µM did not increase cell matrix adhesion over control levels compared to the non-exposed control (p = 0.559). The adhesion of L-NAME-treated cells was not different from the control cell population, and the percent of adherent cells was 62.1% ± 0.8 % (p = 0.452).
Assessment of transmigration five hours later showed that SNAP-induced changes in TM cell migration were not significantly different from the non-treated control cells (Fig. 4). The percent of migrated cells at 1 or 10 µM was 16.3% and 15.3%, respectively. Migrated cells in the controls were 15.6% of the total added cells. The migration of L-NAME-treated cells was not different from the control cell population, and the percent of migrated cells was 18.0%.
In cultured TM cells, the relative level of mRNA expression for four MMPs and two TIMPs in response to SNAP was determined by RT-PCR. Expression of MMP-2 decreased as the concentration of SNAP increased (Fig. 5A). At a concentration of 1 µM, the expression of MMP-2 decreased slightly to an average of 7.5%. At 10 µM, a further dose-dependent decrease in MMP-2 expression was observed (p = 0.041). At 1 µM, the expression of MT1-MMP was unchanged (Fig. 5B). At 10 µM, expression was increased to an average of 12.4% (p = 0.025). Overall, the expression of MMP-2 decreased and MT1-MMP increased in response to 10 µM SNAP.
The expression of MMP-3 decreased at 10 µM, but the decrease was not statistically significant (p = 0.109) (Fig. 6). MMP-3 mRNAs were not detected without IL-1α stimulation. MMP-9 mRNAs were not detected with or without IL-1α stimulation of TM cells.
The expression of TIMP-1 did not change in response to SNAP (Fig. 7A). The expression of TIMP-1 decreased slightly at 10 µM, but the decrease was not statistically significant. At 1 µM, the expression of TIMP-2 increased slightly (Fig. 7B) to an average of 10.5% at 10 µM (p = 0.005). Overall, the expression of TIMP-1 did not change, whereas TIMP-2 expression increased in response to SNAP.
NO has multiple functions on vascular cells including the migration and/or proliferation of cells [29]. The regulation of gene expression and activity of various MMPs is complex and NO seems to play a role. MMP-2 (gelatinase A) activation is suppressed by NO donors in human breast cancer cells. However, NO has been shown to inhibit the expression of MMP-9 (gelatinase B) but not MMP-2 in rat smooth muscle cells [303132]. MMPs degrade the basement membrane and ECM, facilitating cell migration. Among the MMPs, MMP-2 and MMP-9 play a critical role in cell migration [33].
In both ECs and VSMCs, MMP appears to be a key molecular effector of NO during migration [3435]. NO inhibits MMP-2 expression, attenuates endothelial migration, and reversibly inhibits migration independent of proliferation or cytotoxicity in cultured VSMCs. However, there are controversial reports on the effects of NO on cell migration. ECs treated with NO have been shown to have increased migratory activity [36]. In contrast, other studies demonstrated that NO donors increase NO production, which leads to inhibition of endothelial migration [3738].
Numerous MMPs have been detected in TM cells and tissues. In addition, MMP mRNA and protein levels are up-regulated in response to mechanical stretching, elevated pressure, and various cytokines or growth factors. It is known that MMP-2, -14, -15, and -16 are constitutively expressed at relatively high levels in the TM. MMP-1, -3, and -9 are normally expressed at low levels but are massively up-regulated and activated in response to various stimuli [37]. It is known that NO is involved in the regulation of trabecular outflow, and external administration of NO donors results in decreased IOP [394041]. MMPs play a significant role in the regulation of aqueous humor outflow facility by controlling ECM turnover in the TM. NO modulates the expression of MMPs, which may also be important for tissue remodeling characterized by cell proliferation and migration [42]. In TM cells, numerous MMPs have been detected, and MMP mRNA and protein levels are up-regulated in response to mechanical stretching and elevated pressure. However, the detailed molecular mechanisms of how NO affects TM cell migration is still unclear.
Our results demonstrate that NO had no significant effect on the survival of cultured TM cells, in agreement with a previous report [43]. Epithelial cell migration is dependent on NO in some cell types. For example, NO serves as a switch from a stationary to locomoting phenotype in BS-C-1 cells, and exogenous NO has been shown to elicit chemotaxis of neutrophils in vitro [4445]. Previous studies have demonstrated that NO plays a permissive role in endothelin-1-elicited locomotion of ECs [4647]. We performed adhesion and migration assays to determine whether NO induces stimulation of podokinesis in TM cells. Our results suggest that NO did not mediate adherence or migration of TM cells in response to SNAP. Lack of an effect by L-NAME on adherence indicates that TM cells in culture express minimal endothelial nitric oxide synthease activity, and it is possible to elicit an effect by L-NAME only when cells are stimulated. Overall, incubation of TM cells with an NO donor did not affect cell adherence. These findings further support that interference by NO with respect to TM cell migration was negligible. Thus, it appears that NO does not cause significant changes on TM cell adherence or migration, unlike ECs or VSMCs.
Since MMP-2 and MMP-9 are particularly relevant for migration, and MT1-MMP (MMP-14) acts as an MMP-2 activator [933], we focused on these MMPs to evaluate the effect on migration in response to NO. MMP-2 and MT1-MMP mRNA are known to be highly expressed in TM cells. To gain more insight into how MMP activation is regulated in response to NO donors during human TM cell migration, we evaluated the effects of SNAP on the expression of MMPs and TIMPs. We observed that treatment with SNAP resulted in a decrease of MMP-2 expression compared with control cells. Since MT1-MMP has been reported to be a key molecular effector of NO during EC migration [48], we also explored MT1-MMP expression in human TM cells treated with SNAP. We observed a slight increase in MT1-MMP expression compared with control cells.
MMP-1, -3, and -9 are normally expressed at low levels but are massively up-regulated and activated in response to various types of stimuli including IL-1α [4950]. Although IL-1α stimulation was selective for MMP-3, we observed no significant difference in MMP-3 expression in response to SNAP. Furthermore, we did not observe expression of MMP-9 mRNA in control or SNAP-treated TM cells, which is in agreement with a previous study [9]. The absence of MMP-9 and concurrent up-regulation of TIMP-2 along with down-regulation of MMP-2 may explain the limited effect of NO donors on TM cell migration [50]. The results from our experiments show that TIMP-2 activation in TM cells in response to an NO donor occurs despite increased expression of MT1-MMP mRNA. Thus, it is possible that NO has a higher affinity for TIMP-2 [51].
Depletion of TM cells is considered to play a pivotal role within the factors conferring the development of primary open angle glaucoma (POAG). In fact, age-related TM cell loss is even more pronounced in patients with POAG than in age-matched controls. In this context, detachment from the TM and migration from the outflow system stimulated by factors present in the aqueous humor has been suggested as one mechanism conferring meshwork cell depletion in POAG [11121314]. Immunoreactions for MMPs are stronger in POAG samples compared to controls, with the exception of MMP-2 levels. Moreover, the staining intensity of MMP-1 or MMP-3 is significantly higher in POAG samples [5253]. It has been reported that the MMP-2/TIMP-2 ratio is increased in the aqueous humor of POAG eyes [54], possibly as a result of detachment or loss of TM cells. These results suggest that MMP-1 and MMP-3 are the most likely candidates to be involved in the remodeling of trabecular tissue in POAG eyes. It is also likely that cell loss can be explained by local cell death or TM cell migration. Strong attachment to the trabecular ECM enhances inhibition. If a cell enters the cell cycle to replenish the diminishing TM cell population, it will loosen its contact with the ECM. The present study demonstrates that NO elicited a decrease in MMP-2 activity, an absence of MMP-9 activity, and an increase in TIMP-2 activity without a permissive role of NO in TM cell adhesion or migration, which, if occurred, would result in TM cell detachment and subsequent cell loss.
An imbalance in the equilibrium between MMP and TIMP levels may be an important factor in the maintenance and regulation of the ECM in the TM. A decrease in MMP-2 and an increase in TIMP-2 secretion may play an important role in inhibiting cell migration by altering the ratio of MMPs to TIMPs. This shifts the balance of MMP activity, which may favor the inhibition of cell migration. Our data suggest that the MMP-2/TIMP-2 ratio decreased in response to exogenous NO.
In conclusion, we have demonstrated that NO, an IOP-lowering agent, does not significantly affect migration of TM cells. Thus, NO may increase trabecular outflow facility without TM cell loss. In vitro analyses of TM cell migration had the limitation of being remote from physiological and pathological events in vivo. Further in vivo analyses are necessary to confirm our observations.
Figures and Tables
Fig. 1
Effect of S-nitroso-N-acetyl-penicillamine (SNAP) with interleukin-1α (IL-1α) on the production of nitric oxide (NO) in trabecular meshwork (TM) cells. SNAP significantly increased NO production in a dose-dependent manner (*p < 0.05). Production of NO in TM cells incubated with IL-1α was not different compared with the control. Addition of Nω-nitro-L-arginine methyl ester (L) to 10 µM SNAP significantly inhibited NO production (**p < 0.05). Values are presented as means ± standard error of the mean.

Fig. 2
Effect of Nω-nitro-L-arginine methyl ester (L-NAME), interleukin-1α (IL-1α), and S-nitroso-N-acetyl-penicillamine (SNAP) on the survival of trabecular meshwork cells. L-NAME, IL-1α, and SNAP did not have different effects on survival compared with the control (p > 0.05). Values are presented as means ± standard error of the mean.
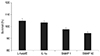
Fig. 3
Effect of Nω-nitro-L-arginine methyl ester (L-NAME) and S-nitroso-N-acetyl-penicillamine (SNAP) on the adhesion of trabecular meshwork cells. L-NAME and SNAP did not have a significant effect on cell adhesion compared with the control (p > 0.05). Values are presented as means ± standard error of the mean.
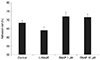
Fig. 4
Effect of Nω-nitro-L-arginine methyl ester (L-NAME) and S-nitroso-N-acetyl-penicillamine (SNAP) on the migration of trabecular meshwork cells. L-NAME and SNAP did not have a significant effect on cell migration significantly compared with the control (p > 0.05). Values are presented as means ± standard error of the mean.
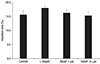
Fig. 5
Effect of S-nitroso-N-acetyl-penicillamine (SNAP) on matrix metalloproteinase (MMP)-2 (A) and MT1-MMP (B) mRNA activity. Trabecular meshwork cells showed a significant decrease in MMP-2 levels and increased MT1-MMP in response to 10 µM SNAP compared to the non-exposed control (*p < 0.05).

Fig. 6
Effect of S-nitroso-N-acetyl-penicillamine (SNAP) on matrix metalloproteinase (MMP)-3 mRNA activity stimulated by interkeukin-1α. Trabecular meshwork cells did not show a significant difference in MMP-3 levels in response to SNAP compared to the non-exposed control (p > 0.05). MMP-9 was not expressed in trabecular meshwork cells after stimulation with interkeukin-1α.
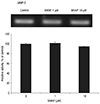
Fig. 7
Effect of S-nitroso-N-acetyl-penicillamine (SNAP) on tissue inhibitor of metalloproteinase (TIMP)-1 (A) and TIMP-2 (B) mRNA activity. Trabecular meshwork cells did not show a significant difference in TIMP-1 levels (p > 0.05) but showed a significant increase in TIMP-2 levels in response to 10 µM SNAP compared to the non-exposed control (*p < 0.05).

References
1. Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009; 88:671–675.
2. Bradley JM, Kelley MJ, Zhu X, et al. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001; 42:1505–1513.
3. Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008; 86:543–561.
4. Alexander JP, Samples JR, Van Buskirk EM, Acott TS. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest Ophthalmol Vis Sci. 1991; 32:172–180.
5. Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003; 22:435–463.
6. Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003; 77:757–765.
7. Lo WR, Rowlette LL, Caballero M, et al. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003; 44:473–485.
8. Vittal V, Rose A, Gregory KE, et al. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005; 46:2857–2868.
9. Oh DJ, Martin JL, Williams AJ, et al. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47:3887–3895.
10. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39:2649–2658.
11. Grierson I, Hogg P. The proliferative and migratory activities of trabecular meshwork cells. Prog Retin Eye Res. 1995; 15:33–67.
12. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981; 21:714–727.
13. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1987; 1(Pt 2):204–210.
14. Grierson I, Wang Q, McMenamin PG, Lee WR. The effects of age and antiglaucoma drugs on the meshwork cell population. Res Clin Forums. 1981; 4:69–92.
15. Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993; 22 Suppl 4:S1–S14.
16. Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999; 43:580–594.
17. Newby AC, George SJ. Proliferation, migration, matrix turnover, and death of smooth muscle cells in native coronary and vein graft atherosclerosis. Curr Opin Cardiol. 1996; 11:574–582.
18. Trachtman H, Futterweit S, Garg P, et al. Nitric oxide stimulates the activity of a 72-kDa neutral matrix metalloproteinase in cultured rat mesangial cells. Biochem Biophys Res Commun. 1996; 218:704–708.
19. Murohara T, Witzenbichler B, Spyridopoulos I, et al. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999; 19:1156–1161.
20. Ziche M, Morbidelli L, Masini E, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994; 94:2036–2044.
21. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells .I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–1049.
22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
23. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138.
24. Okayama N, Ichikawa H, Coe L, et al. Exogenous NO enhances hydrogen peroxide-mediated neutrophil adherence to cultured endothelial cells. Am J Physiol. 1998; 274(5 Pt 1):L820–L826.
25. Okouchi M, Okayama N, Shimizu M, et al. High insulin exacerbates neutrophil-endothelial cell adhesion through endothelial surface expression of intercellular adhesion molecule-1 via activation of protein kinase C and mitogen-activated protein kinase. Diabetologia. 2002; 45:556–559.
26. Hogg P, Calthorpe M, Batterbury M, Grierson I. Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci. 2000; 41:1091–1098.
27. Sato H, Kinoshita T, Takino T, et al. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996; 393:101–104.
28. Oh DJ, Martin JL, Williams AJ, et al. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci. 2006; 47:953–963.
29. Maulik N, Engelman DT, Watanabe M, et al. Nitric oxide/carbon monoxide: a molecular switch for myocardial preservation during ischemia. Circulation. 1996; 94:9 Suppl. II398–II406.
30. Eberhardt W, Beeg T, Beck KF, et al. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000; 57:59–69.
31. Zhang HJ, Zhao W, Venkataraman S, et al. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002; 277:20919–20926.
32. Upchurch GR Jr, Ford JW, Weiss SJ, et al. Nitric oxide inhibition increases matrix metalloproteinase-9 expression by rat aortic smooth muscle cells in vitro. J Vasc Surg. 2001; 34:76–83.
33. Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006; 69:614–624.
34. Chen HH, Wang DL. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol Pharmacol. 2004; 65:1130–1140.
35. Sarkar R, Meinberg EG, Stanley JC, et al. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996; 78:225–230.
36. Kawasaki K, Smith RS Jr, Hsieh CM, et al. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003; 23:5726–5737.
37. Lau YT, Ma WC. Nitric oxide inhibits migration of cultured endothelial cells. Biochem Biophys Res Commun. 1996; 221:670–674.
38. Kook H, Ahn KY, Lee SE, et al. Nitric oxide-dependent cytoskeletal changes and inhibition of endothelial cell migration contribute to the suppression of angiogenesis by RAD50 gene transfer. FEBS Lett. 2003; 553:56–62.
39. Matsuo T. Basal nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–635.
40. Haefliger IO, Dettmann E, Liu R. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999; 58:99–105.
41. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58:99–105.
42. Pfeilschifter J, Eberhardt W, Huwiler A. Nitric oxide and mechanisms of redox signalling: matrix and matrix-metabolizing enzymes as prime nitric oxide targets. Eur J Pharmacol. 2001; 429:279–286.
43. Kim JW, Heo H, Lee HW. Effect of nitric oxide on the proliferation of cultured porcine trabecular meshwork cells. Korean J Ophthalmol. 2003; 17:1–6.
44. Noiri E, Peresleni T, Srivastava N, et al. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. Am J Physiol. 1996; 270(3 Pt 1):C794–C802.
45. Beauvais F, Michel L, Dubertret L. Exogenous nitric oxide elicits chemotaxis of neutrophils in vitro. J Cell Physiol. 1995; 165:610–614.
46. Noiri E, Lee E, Testa J, et al. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol. 1998; 274(1 Pt 1):C236–C244.
47. Noiri E, Hu Y, Bahou WF, et al. Permissive role of nitric oxide in endothelin-induced migration of endothelial cells. J Biol Chem. 1997; 272:1747–1752.
48. Genis L, Gonzalo P, Tutor AS, et al. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood. 2007; 110:2916–2923.
49. Samples JR, Alexander JP, Acott TS. Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone. Invest Ophthalmol Vis Sci. 1993; 34:3386–3395.
50. Pang IH, Hellberg PE, Fleenor DL, et al. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:3485–3493.
51. Ailenberg M, Silverman M. Cellular activation of mesangial gelatinase A by cytochalasin D is accompanied by enhanced mRNA expression of both gelatinase A and its membrane-associated gelatinase A activator (MT-MMP). Biochem J. 1996; 313(Pt 3):879–884.
52. Ronkko S, Rekonen P, Kaarniranta K, et al. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2007; 245:697–704.
53. Keller KE, Aga M, Bradley JM, et al. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88:676–682.
54. Schlotzer-Schrehardt U, Lommatzsch J, Kuchle M, et al. Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003; 44:1117–1125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download