Abstract
Purpose
Methods
Results
Figures and Tables
Fig. 1
(A) A total of 50 neovascular age-related macular degeneration (nAMD) patients visited our clinic, and 27 patients were excluded. (B) All patients in this study underwent a complete ophthalmologic examination, including an assessment of best-corrected visual acuity (BCVA), spectral domain optical coherence tomography (SD-OCT), fluorescein angiography (FA), indocyanine green angiography (ICGA) and fundus autofluorescence (FAF) imaging before the first intravitreal anti-vascular endothelial growth factor injection (pre-treatment) and BCVA, SD-OCT, and FA 1 month after the initial three intravitreal anti-vascular endothelial growth factor injections. Post-treatment gray scale of FAF (%) was measured in the test that was performed 1 month after the last injection (post-treatment). PED = pigment epithelial detachment; SRH = subretinal hemorrhage; RAP = retinal angiomatous proliferation; FU = follow-up; CNV = choroidal neovascularization.

Fig. 2
Gray scale measurements of fundus autofluorescence (FAF) images for choroidal neovascularization (CNV) areas delineated in fluorescein angiography (FA) and spectral domain optical coherence tomography (SD-OCT) were analyzed using the ImageJ program. (A) FAF image of CNV. White line indicates the boundary of CNV. (B) FA image of CNV. White line indicates the boundary of CNV. (C) SD-OCT image of CNV. White vertical lines indicate the diameter of CNV.
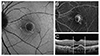
Fig. 3
Changes in fundus autofluorescence and spectral domain optical coherence tomography (SD-OCT) after intravitreal anti-vascular endothelial growth factor injection in an exudative age-related macular degeneration patient with type 1 choroidal neovascularization (CNV). After treatment, as subretinal fluid was absorbed as shown on SD-OCT, the brightness of autofluorescence in the area of CNV was increased. (A) Fundus autofluorescence of pre-treatment CNV. Initial best-corrected visual acuity (BCVA) was 20 / 100. (B) SD-OCT of pre-treatment CNV. CNV with subretinal fluid was seen. (C) Fundus autofluorescence of post-treatment CNV. Final BCVA was 20 / 60. (D) Post-treatment SD-OCT. Subretinal fluid was completely absorbed.
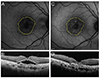
Fig. 4
Changes in fundus autofluorescence and spectral domain optical coherence tomography (SD-OCT) after intravitreal anti-vascular endothelial growth factor injection in an exudative age-related macular degeneration patient with type 2 choroidal neovascularization (CNV). After treatment, CNV size was decreased and macular edema disappeared. Also, the brightness of fundus autofluorescence was increased after treatment. (A) Fluorescein angiography of pre-treatment type 2 CNV patient. (B) Fundus autofluorescence of pre-treatment type 2 CNV patient. (C) SD-OCT image of pre-treatment type 2 CNV patient. CNV, intraretinal fluid, and subretinal fluid were seen. (D) Fundus autofluorescence of post-treatment type 2 CNV patient. The arrow indicates increased fundus autofluorescence in CNV area. (E) SD-OCT image of post-treatment type 2 CNV patient. CNV, intraretinal fluid, and subretinal fluid were absorbed.
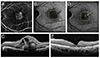
Table 1
Baseline characteristics of patients

Values are presented as the mean ± standard deviation.
CNV = choroidal neovascularization; logMAR = logarithm of the minimum angle of resolution; SD-OCT = spectral domain optical coherence tomography; IS/OS = photoreceptor inner and outer segment junction; ELM = external limiting membrane; CMT = central macular thickness.
*Chi-square test; †Mann-Whitney test.
Table 3
Changes in VA and SD-OCT parameters before and after treatment

Values are presented as the mean ± standard deviation; Wilcoxon signed rank test, p < 0.05.
VA = visual acuity; SD-OCT = spectral domain optical coherence tomography; CNV = choroidal neovascularization; logMAR = logarithm of the minimum angle of resolution; IS/OS = photoreceptor inner and outer segment junction; ELM = external limiting membrane; CMT = central macular thickness.
Table 4
Correlation of post VA (logMAR) with pre and post spectral domain optical coherence tomography parameters

Table 5
Correlation of post fundus autofluorescence with pre and post SD-OCT parameters and VA

Spearman rank correlation, p < 0.05.
SD-OCT = spectral domain optical coherence tomography; VA = visual acuity; CNV = choroidal neovascularization; logMAR = logarithm of the minimum angle of resolution; IS/OS = the junction between the inner and outer segments of the photoreceptors; ELM = external limiting membrane; CMT = central macular thickness.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download