Abstract
Purpose
To investigate the therapeutic effects of mineral oil (MO) and hyaluronic acid (HA) mixture eye drops on the tear film and ocular surface in a mouse model of experimental dry eye (EDE).
Methods
Eye drops consisting of 0.1% HA alone or mixed with 0.1%, 0.5%, or 5.0% MO were applied to desiccating stress-induced murine dry eyes. Tear volume, corneal irregularity score, tear film break-up time (TBUT), and corneal fluorescein staining scores were measured at 5 and 10 days after treatment. Ten days after treatment, goblet cells in the conjunctiva were counted after Periodic acid-Schiff staining.
Results
There was no significant difference in the tear volume between desiccating stress-induced groups. The corneal irregularity score was lower in the 0.5% MO group compared with the EDE and HA groups. The 0.5% and 5.0% MO groups showed a significant improvement in TBUT compared with the EDE group. Mice treated with 0.1% and 0.5% MO mixture eye drops showed a significant improvement in fluorescein staining scores compared with the EDE group and the HA group. The conjunctival goblet cell count was higher in the 0.5% MO group compared with the EDE group and HA group.
Conclusions
The MO and HA mixture eye drops had a beneficial effect on the tear films and ocular surface of murine dry eye. The application of 0.5% MO and 0.1% HA mixture eye drops could improve corneal irregularity, the corneal fluorescein staining score, and conjunctival goblet cell count compared with 0.1% HA eye drops in the treatment of EDE.
Dry eye disease is a multifactorial disorder of the tears and ocular surface [1]. It leads to various ocular symptoms and tear film instability induce potential damage of the ocular surface and ocular surface inflammation [1]. The resulting diminished vision and quality of life affect millions of people worldwide [2]. Treatments for dry eye disease in increasing order of severity include artificial tears, anti-inflammatory agents, immunosuppressants, punctal plugs, serum eye drops, contact lenses, and surgery [3,4]. Artificial tears are typically used as the first-line management of dry eye disease. They improve the stability of the tear film and provide symptomatic relief [5].
Hyaluronic acid (HA) is a linear polymer composed of long chains of repeating disaccharide units of N-acetylglucosamine and glucuronic acid [6]. HA is an avid moisturizer and has a higher residency time than other artificial tear components. In addition, HA can protect the ocular surface epithelium by facilitate epithelial healing [7]. 0.1% HA eye drops are commonly used to treat dry eye disease and were found to be effective in improving symptoms as well as signs including corneal epithelial injury [8,9,10]. However, due to a short duration of action and deficient tear lipid or mucin components, HA eye drops have several limitations including the need for frequent application.
Mineral oil (MO) is a complex mixture of saturated hydrocarbons derived from petroleum through various refining steps and subsequent purification [11]. MO-based artificial tears as an ointment formulation prolongs tear retention time in the eye, therefore requiring fewer daily applications [12]. However, no study has been performed on eye drop mixtures of HA and MO as an oil component for dry eye.
The purpose of this study was to evaluate the efficacy of MO and HA combination eye drops for the management of dry eye disease using a desiccating stress-induced mouse model, by evaluating the changes of tear production, tear film break-up time (TBUT), fluorescein staining, ocular surface irregularities, and goblet cell count in the conjunctiva.
The research protocol was approved by the Chonnam National University Medical School Research Institutional Animal Care and Use Committee. All animals were treated according to the standards in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Six- to eight-week-old female C57BL/6 mice were used in these experiments.
Experimental dry eye (EDE) was induced by subcutaneous injection of 0.5 mg/0.2 mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO, USA) four times a day (9 a.m., 1 p.m., 5 p.m., and 9 p.m.) with exposure to an air draft and 30% ambient humidity [13,14,15,16]. During these experiments, the animal's behavior, food, and water intake were not restricted.
The mice were randomly divided into six groups (n = 5 per group) depending on topical treatment administered: untreated (UT) mice; EDE mice that received no eye drops; EDE mice treated with non-preservative 0.1% HA eye drops (Alcon Korea, Seoul, Korea); EDE mice treated with 0.1% MO and 0.1% HA mixture eye drops; EDE mice treated with 0.5% MO and 0.1% HA mixture eye drops; and EDE mice treated with 5.0% MO and 0.1% HA mixture eye drops. MO and HA mixture eye drops were created by mixing MO with 0.1% HA using a surfactant. All treatment groups received 2 µL of eye drops four times a day.
At 5 and 10 days after treatment, tear volume, corneal irregularity score, TBUT, and corneal fluorescein staining score were measured two hours after application of the last eye drops. Ten days after treatment, the mice were euthanized, and Periodic acid-Schiff staining was performed. Each experiment was repeated three times.
Tear volume was assessed using phenol-red impregnated Zone-Quick cotton threads (Oasis, Glendora, CA, USA) as previously described [17,18,19]. Cotton threads were placed in the lateral canthus for 20 seconds. The threads length that became wet by tears was measured using a SMZ 1500 stereoscopic zoom microscope (Nikon, Melville, New York, NY, USA). A standard curve was derived to convert distance into volume.
The severity of the corneal surface irregularity was graded by measuring the distortion of a white ring from the fiber-optic ring illuminator of the stereoscopic zoom microscope by two blinded observers [18,19]. The corneal irregularity score was calculated using a 6-point scale (0-5) based on the number of distorted quarters in the reflected ring, as follows: 0, no distortion; 1, distortion in one quarter of the ring; 2, distortion in two quarters; 3, distortion in three quarters; 4, distortion in all four quadrants; and 5, severe distortion, in which no ring could be recognized [20].
One micro-liter of 0.1% liquid sodium fluorescein was gently applied to the conjunctival sac. After 3 blinks, the interval between the last complete blink and the appearance of the first corneal black spot was recorded in seconds using a slit-lamp microscope equipped with a cobalt blue filter. And the mean of three measurements was calculated [21,22].
The severity of corneal epithelial damage was graded by two blinded observers who measured the fluorescein staining of the mouse cornea. After 0.1% liquid sodium fluorescein was dropped into the conjunctival sac, the corneal epithelial damage was graded with a slit-lamp microscope equipped with a cobalt blue filter. The fluorescein staining score was calculated using a 5-point scale (0-4), as follows: 0, no fluorescein staining; 1, slightly punctuate staining <30 spots; 2, punctuate staining >30 spots, but not diffuse; 3, severe diffuse staining but no positive plaque; and 4, severe diffuse staining with positive fluorescein plaque [22]. After scoring all four corneal quadrants, the total score was averaged.
Eyes and adnexa were surgically excised, fixed in 4% paraform-aldehyde, and embedded in paraffin. Six-micrometer sections were stained with Periodic acid-Schiff reagent. Sections from each group were examined and photographed with a microscope (BX53; Olympus, Tokyo, Japan) equipped with a digital camera (F2; Foculus, Finning, Germany). Goblet cells in the conjunctiva were counted in three sections from each eye using image analysis software (Media Cybernetics, Silver Spring, MD, USA) and expressed as the number of goblet cells per 100 µm [18,19].
Statistical differences in the tear volume, corneal irregularity score, TBUT, and fluorescein staining score were evaluated by one-way analysis of variance, with post hoc analysis. Kruskal-Wallis and Mann-Whitney tests were used to compare the levels of cytokines and chemokine between different groups. A p-value <0.05 was considered statistically significant.
The mean tear volumes at 5 days after desiccating stress were 0.035 ± 0.004 µL in the UT group, 0.013 ± 0.003 µL in the EDE group, 0.014 ± 0.002 µL in the HA group, 0.013 ± 0.002 µL in the mixed 0.1% MO group, 0.017 ± 0.003 µL in the mixed 0.5% MO group, and 0.013 ± 0.002 µL in the mixed 5.0% MO group. There were no significant differences in the tear volumes between groups. At 10 days, the mean volume in all groups showed similar finding to those at 5 days.
Corneal irregularity scores increased from 0.25 ± 0.45 to 3.92 ± 0.90 (p < 0.01) at 5 days after desiccating stress. The mean corneal irregularity scores at 5 days after treatment were 3.46 ± 0.52 in the HA group (p = 0.51 compared with the EDE group), 3.36 ± 0.51 in the mixed 0.1% MO group (p = 0.30 vs. the EDE group; p = 0.99 vs. the HA group), 1.27 ± 0.47 in the mixed 0.5% MO group (p < 0.01 vs. the EDE and HA groups), and 3.27 ± 0.79 in the mixed 5.0% MO group ( p = 0.16 vs. the EDE group; p = 0.98 vs. the HA group). The results for corneal irregularity scores in all groups at 10 days after desiccating stress were similar to those at 5 days (Fig. 1).
In the UT group, TBUT was 4.13 ± 0.58 and 3.75 ± 1.04 seconds at 5 and 10 days respectively. After desiccating stress, the mean TBUT in the EDE group was 1.00 ± 1.04 and 1.26 ± 0.54 seconds at 5 and 10 days, respectively (p < 0.05 compared with the UT group for both). In the treatment group, the TBUT values at 5 days were 1.76 ± 1.04 seconds in the HA group (p = 0.31 vs. the EDE group), 1.67 ± 1.16 seconds in the mixed 0.1% MO group (p = 0.40 vs. the EDE group; p = 0.10 vs. the HA group), 2.13 ± 0.60 seconds in the mixed 0.5% MO group (p = 0.02 vs. the EDE group; p = 0.93 vs. the HA group), and 2.10 ± 0.76 seconds in the mixed 5.0% MO group (p = 0.01 vs. the EDE group; p = 0.93 vs. the HA group). At 10 days, TBUT in all groups showed similar findings to those at 5 days (Fig. 2).
After desiccating stress, corneal fluorescein staining scores at 5 and 10 days were 3.00 ± 0.93 and 2.86 ± 0.64 in the EDE group (p < 0.01 compared with the UT group for both). After treatment, the mean staining scores were 2.10 ± 0.32 in the HA group (p < 0.01 vs. the EDE group), 1.27 ± 0.47 in the mixed 0.1% MO group (p < 0.01 vs. the EDE group; p = 0.03 vs. the HA group), 1.18 ± 0.60 in the mixed 0.5% MO group (p < 0.01 vs. the EDE group; p = 0.01 vs. the HA group), 3.33 ± 0.49 seconds in the mixed 5.0% MO group ( p = 0.67 vs. the EDE group; p < 0.01 vs. the HA group). The results for corneal fluorescein staining scores in all groups at 10 days after desiccating stress were similar to those at 5 days (Fig. 3).
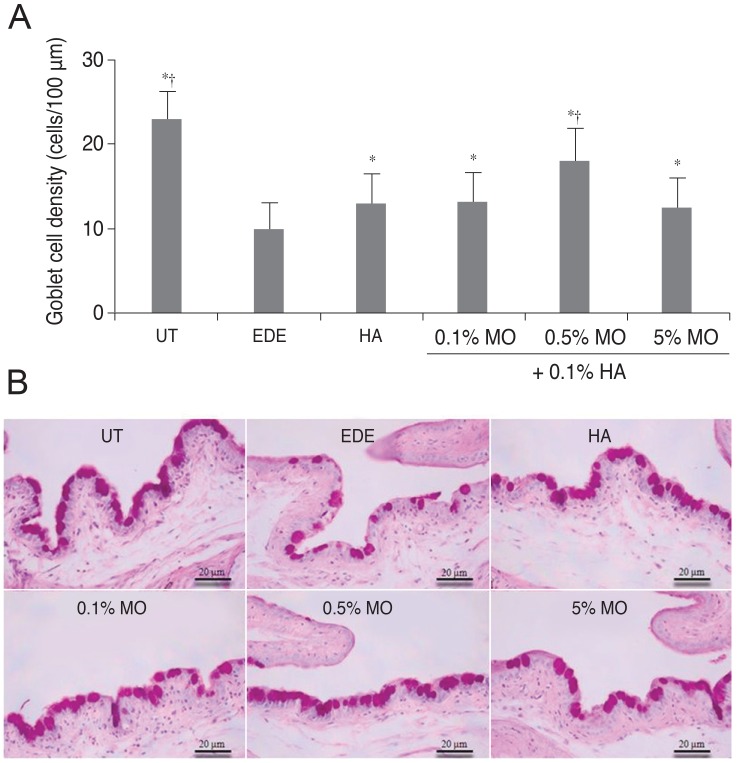
The mean conjunctival goblet cell count significantly decreased in the EDE group (10.0 ± 0.8 cells/100 µm) compared with the UT group (23.0 ± 1.4 cells/100 µm) (p < 0.01). The mean goblet cell counts were 13.0 ± 1.41 cells/100µm in the HA group (p = 0.02 vs. the EDE group), 13.3 ± 0.96 cells/100 µm in the mixed 0.1% MO group (p < 0.01 vs. the EDE group; p = 0.78 vs. the HA group), 18.0 ± 0.82 cells/100 µm in the mixed 0.5% MO group (p < 0.01 vs. the EDE group; p = 0.02 vs. the HA group), and 12.5 ± 1.29 cells/100 µm in the mixed 5.0% MO group (p = 0.02 vs. the EDE group; p = 0.62 vs. the HA group) (Fig. 4).
HA can be found naturally in all vertebrates in the extracellular matrix of the skin, synovial fluid, and vitreous body of the eye. It is a biopolymer of disaccharide units composed of N-acetylglucosamine and glucuronic acid in linear chains of varying molecular weights [23]. By forming a protective coating that help prevent further irritation and d amage of the corneal epithelium, HA can improve corneal fluorescein staining [24]. In addition, dose-dependent ability of HA to decrease the size of the wound area has been reported [25]. Topical use of HA provides both objective and subjective symptomatic relief in patients with dry eye disease [8,9,10]. In addition, there is evidence that hyaluronate may play a role in controlling the localized inflammation of ocular surface in patients with keratoconjuctivitis sicca [26].
HA has several biological effects that are exerted through its direct interaction with a cell surface receptor (CD44). The effects include metastatic potential of tumor cells, secretion of cytokines and chemokines, mediation of inflammatory responses, and cell division [27,28,29]. In addition, binding of HA to CD44 enhances the growth of the corneal epithelial cells and promotes migration of human corneal epithelial cells [27,28]. The expression of CD44, the receptor of HA, can be increased in corneal and conjunctival cells of patients with dry eye disease, whereas it is decreased following the use of HA eye drops [24,30].
Different types of artificial tear formulation, including gel-based, cellulose-based, carbomer-based and MO-based, have been developed to relieve symptoms of dry eye and have been designed as alternatives to classic artificial tear formulation [12,31,32]. Among these new formulations, MO-based formulations can prolong retention times compared with aqueous tear substitutes, but they may cause a sticky sensation and blurred vision [33]. One study demonstrated that a preservative-free oil-in-water emulsion containing 7% soy bean oil, 3% egg yolk phospholipids, and1.8% glycerol could relieve and improve clinical signs such as tear volume and corneal fluorescent staining in a mouse model of dry eye [34]. In addition, MO-based artificial tears were as effective as cellulose-based and carbomer-based artificial tears in reducing subjective symptoms, and objective signs including TBUT [12].
In the present study, we evaluated the effect of mixed 0.1% HA and MO eye drops on tear production, ocular surface irregularity, TBUT, corneal fluorescein staining score, and conjunctival goblet cell count in an EDE model. There was no significant difference of tear volume between the stress-induced groups. The corneal irregularity score was lower in the mixed 0.5% MO group compared with the EDE and HA groups. The mixed 0.5% and 5.0% MO groups showed a significant improvement in TBUT compared with the EDE group. In the mixed 0.1% and 0.5% MO groups, there was a significant improvement of the corneal fluorescein staining score compared with the EDE or HA groups. The conjunctival goblet cell count was higher in the mixed 0.5% MO group compared with the EDE group and the HA group.
Our results showed that HA eye drops containing MO could have a beneficial effect on the tear film and ocular surface of EDE. These results may be attributed to a synergetic effect of HA and MO. The effect of HA as artificial tears which could decrease corneal fluorescein staining by promoting epithelial healing might be enhanced by the addition of MO, and MO itself as a lipid component might improve tear stability and corneal surface irregularity by prolonging the retention time of the tear film. The 0.1% MO showed an improvement of corneal fluorescein staining scores and goblet cell count, and the 5.0% MO improved the TBUT and goblet cell count, while the 0.5% MO ameliorated all measured parameters. These results suggested that the application of a mixture of 0.5% MO and HA eye drops was the best combination for the treatment of EDE compared with the other MO groups.
The application of MO eye drops, however, may cause a sticky, burning sensation and blurred vision [33]. Because this study is based on the dry eye mouse model, further studies based on human will be needed to examine any possible side effects.
In conclusion, the application of eye drops containing a mixture of 0.5% MO and 0.1% HA was more effective in corneal irregularity score, corneal fluorescein staining score, and conjunctival goblet cell count on the ocular surface compared with 0.1% HA eye drops in a mouse model of dry eye. These results showed that the MO and HA mixture was more effective than HA agents alone for treating dry eye associated with ocular surface damage. In the near future, clinical studies on the safety and efficacy of MO and HA mixture eye drops for the treatment of dry eye will be needed.
Acknowledgements
The study was partially supported by the Chonnam National University Hospital Biomedical Research Institute (CRI 11076-21 and CRI 13906-22) and Chong Kun Dang Pharmaceutical Co., Seoul, Korea.
References
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92. PMID: 17508116.
2. Cuevas M, Gonzalez-Garcia MJ, Castellanos E, et al. Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by Meibomian gland dysfunction (MGD). Curr Eye Res. 2012; 37:855–863. PMID: 22632103.

3. Gupta H, Jain S, Mathur R, et al. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007; 14:507–515. PMID: 18027180.

4. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25:900–907. PMID: 17102664.
5. McCann LC, Tomlinson A, Pearce EI, Papa V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea. 2012; 31:1–5. PMID: 21968605.

6. McDonald CC, Kaye SB, Figueiredo FC, et al. A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye (Lond). 2002; 16:601–607. PMID: 12194076.

7. Wysenbeek YS, Loya N, Ben Sira I, et al. The effect of sodium hyaluronate on the corneal epithelium. An ultrastructural study. Invest Ophthalmol Vis Sci. 1988; 29:194–199. PMID: 3338879.
8. Sand BB, Marner K, Norn MS. Sodium hyaluronate in the treatment of keratoconjunctivitis sicca A double masked clinical trial. Acta Ophthalmol (Copenh). 1989; 67:181–183. PMID: 2658462.
9. DeLuise VP, Peterson WS. The use of topical Healon tears in the management of refractory dry-eye syndrome. Ann Ophthalmol. 1984; 16:823–824. PMID: 6508097.
10. Stuart JC, Linn JG. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann Ophthalmol. 1985; 17:190–192. PMID: 3873200.
11. Rawlings AV, Lombard KJ. A review on the extensive skin benefits of mineral oil. Int J Cosmet Sci. 2012; 34:511–518. PMID: 22994201.

12. Wang IJ, Lin IC, Hou YC, Hu FR. A comparison of the effect of carbomer-, cellulose- and mineral oil-based artificial tear formulations. Eur J Ophthalmol. 2007; 17:151–159. PMID: 17415686.

13. Yoon KC, De Paiva CS, Qi H, et al. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008; 30:212–221. PMID: 17988834.

14. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006; 176:3950–3957. PMID: 16547229.
15. De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006; 83:526–535. PMID: 16643899.

16. De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007; 48:2553–2560. PMID: 17525184.
17. Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006; 32:272–276. PMID: 17099387.

18. Li Z, Choi W, Oh HJ, Yoon KC. Effectiveness of topical infliximab in a mouse model of experimental dry eye. Cornea. 2012; 31(Suppl 1):S25–S31. PMID: 23038030.

19. Li Z, Woo JM, Chung SW, et al. Therapeutic effect of topical adiponectin in a mouse model of desiccating stress-induced dry eye. Invest Ophthalmol Vis Sci. 2013; 54:155–162. PMID: 23211823.

20. De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006; 47:2847–2856. PMID: 16799024.

21. Dogru M, Erturk H, Shimazaki J, et al. Tear function and ocular surface changes with topical mitomycin (MMC) treatment for primary corneal intraepithelial neoplasia. Cornea. 2003; 22:627–639. PMID: 14508259.

22. Xiao X, He H, Lin Z, et al. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Invest Ophthalmol Vis Sci. 2012; 53:191–197. PMID: 22159022.

23. Vogel R, Crockett RS, Oden N, et al. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena). Am J Ophthalmol. 2010; 149:594–601. PMID: 20346777.

24. Brignole F, Pisella PJ, Dupas B, et al. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol. 2005; 243:531–538. PMID: 15965673.

25. Nakamura M, Hikida M, Nakano T. Concentration and molecular weight dependency of rabbit corneal epithelial wound healing on hyaluronan. Curr Eye Res. 1992; 11:981–986. PMID: 1451529.

26. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–589. PMID: 9820935.
27. Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord. 2005; 5:3–14. PMID: 15720220.

28. Turino GM, Cantor JO. Hyaluronan in respiratory injury and repair. Am J Respir Crit Care Med. 2003; 167:1169–1175. PMID: 12714341.

30. Lerner LE, Schwartz DM, Hwang DG, et al. Hyaluronan and CD44 in the human cornea and limbal conjunctiva. Exp Eye Res. 1998; 67:481–484. PMID: 9820796.

31. Sullivan LJ, McCurrach F, Lee S, et al. Efficacy and safety of 0.3% carbomer gel compared to placebo in patients with moderate-to-severe dry eye syndrome. Ophthalmology. 1997; 104:1402–1408. PMID: 9307633.

32. Wang TJ, Wang IJ, Ho JD, et al. Comparison of the clinical effects of carbomer-based lipid-containing gel and hydroxypropyl-guar gel artificial tear formulations in patients with dry eye syndrome: a 4-week, prospective, open-label, randomized, parallel-group, noninferiority study. Clin Ther. 2010; 32:44–52. PMID: 20171410.

34. Scifo C, Barabino S, De Pasquale G, et al. Effects of a new lipid tear substitute in a mouse model of dry eye. Cornea. 2010; 29:802–806. PMID: 20489574.

Fig. 1
Mean corneal irregularity scores (A) and representative figure (B) in the untreated (UT), experimental dry eye (EDE), hyaluronic acid (HA), mixed 0.1% mineral oil (MO), mixed 0.5% MO, and mixed 5.0% MO groups at 5 and 10 days after desiccating stress. *p < 0.05 compared with the EDE group; †p < 0.05 compared with the HA group.
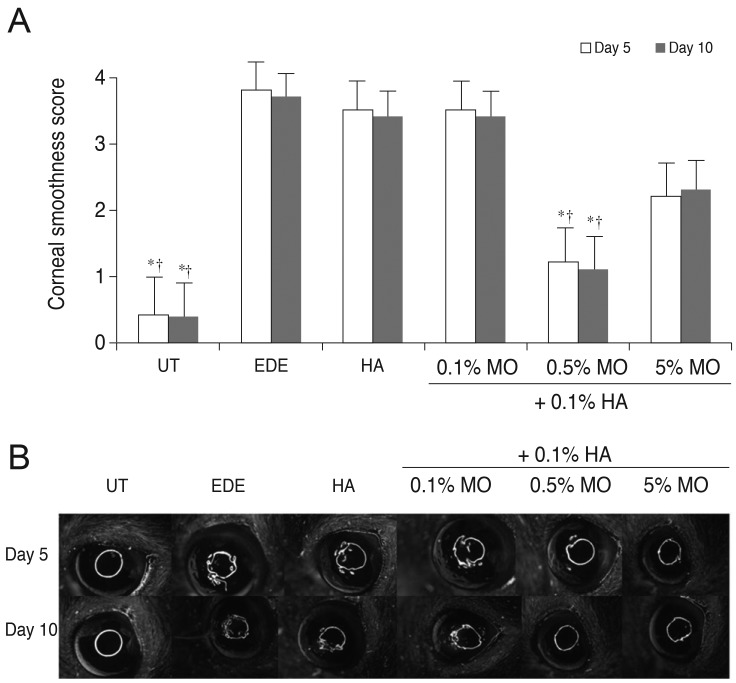
Fig. 2
Tear film break-up time in the untreated (UT), experimental dry eye (EDE), hyaluronic acid (HA), mixed 0.1% mineral oil (MO), mixed 0.5% MO, and mixed 5.0% MO groups at 5 and 10 days after desiccating stress. *p < 0.05 compared with the EDE group; †p < 0.05 compared with the HA group.
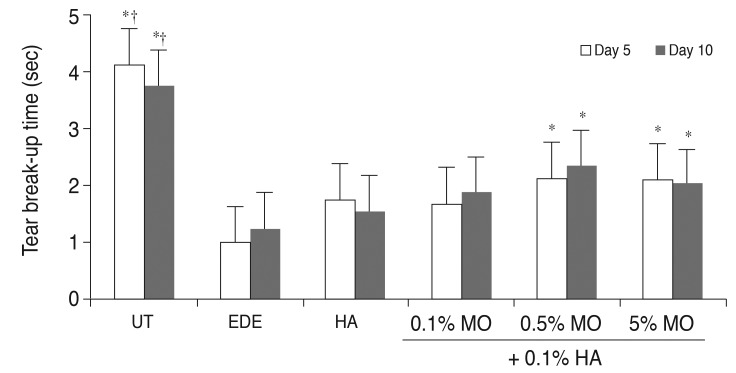
Fig. 3
Corneal fluorescein staining scores (A) and representative figure (B) in the untreated (UT), experimental dry eye (EDE), hyaluronic acid (HA), mixed 0.1% mineral oil (MO), mixed 0.5% MO, and mixed 5.0% MO groups at 5 and 10 days after desiccating stress. *p < 0.05 compared with the EDE group; †p < 0.05 compared with the HA group.
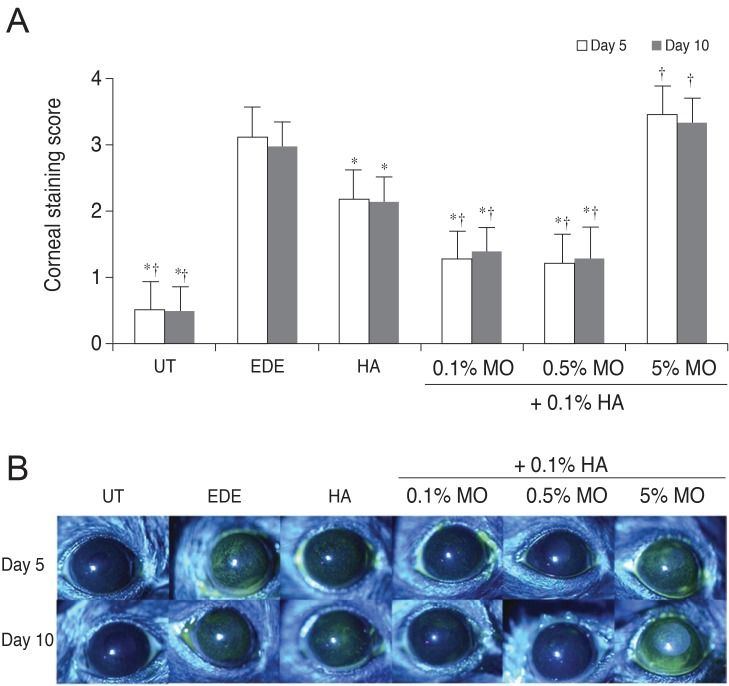
Fig. 4
Mean goblet cell count (A) and representative figures (B) of the untreated (UT), experimental dry eye (EDE), hyaluronic acid (HA), mixed 0.1% mineral oil (MO), mixed 0.5% MO, and mixed 5.0% MO groups at 10 days after desiccating stress. *p < 0.05 compared with the EDE group; †p < 0.05 compared with the HA group. Sections were stained with periodic acid-Schiff.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download