Abstract
Purpose
To investigate the biometric risk factors for corneal surface complications associated with hydrogel soft contact lens (SCL) fitting in myopic patients in Korea.
Methods
This is a retrospective case-control study. The records of 124 subjects (124 eyes) who wore SCLs on a daily basis were reviewed. Thirty-one patients (31 eyes) who were diagnosed with corneal neovascularization (NV) while wearing SCLs were included in the complication group. Ninety-three age- and sex-matched patients (93 eyes) who wore SCLs, who did not have corneal NV and who visited our clinic for correction of refractive errors were included in the control group. The degree of spherical equivalent, astigmatism and corneal base curve radius (BCR) were compared in both groups.
Results
Patients with NV exhibited poorer best corrected visual acuity and more myopia than controls (p = 0.008 and 0.006, respectively). In univariate analysis, highly myopic patients (-9 diopters [D] or higher) were more likely to experience NV (odds ratio [OR], 2.232; 95% confidence interval [CI], 1.602 to 3.105). High astigmatism (≥2 D) increased the risk of complications (OR, 2.717; 95% CI, 1.141 to 6.451). Steep cornea, in which BCR was <7.5 mm, also raised the risk of complications (OR, 4.000; 95% CI, 1.661 to 9.804). Flat cornea was not a risk factor for the development of NV.
Contact lenses are used in the day-to-day life of 120 million people worldwide, and are the most popular method of obtaining refractive correction [1]. Contact lens-related complications can be developed anywhere around the contact lens, including the eyelids, conjunctiva, cornea, adnexa, and even the tear film [2]. Many studies have reported various adverse events, such as infectious keratitis, inflammatory infiltrates, superficial corneal keratitis, papillary conjunctivitis, dry eye, and neovascularization (NV) [3,4,5].
The following risk factors are associated with these adverse events: lens care system, contact lens material, durability, wear schedule, lens power, contact lens solution, and patient-related factors such as smoking [6,7,8,9,10,11,12]. However, biometric risk factors have yet to be fully determined.
Corneal NV afflicts soft contact lens (SCL) wearers, suggesting limbal stem cell damage due to long-term hypoxia or toxic lens solutions [13,14]. However, risk factors involving hypoxic damage have not been previously identified. The authors of the present study hypothesized that corneal biometric factors for SCL fitting as well as intrinsic lens factors such as material properties (oxygen transmissibility) influence NV. High myopia and high astigmatism may affect the peripheral thickness of hydrogel SCLs, reducing peripheral oxygen transmissibility and increasing peripheral mechanical friction. Inappropriate lens-corneal alignment, due to a too-flat or too-steep cornea, may also have an effect on peripheral hypoxic or mechanical damage in a wear of SCLs in limited base curve radius.
Therefore, the present study aimed to investigate whether intrinsic biometric parameters used to determine the fit of SCLs may be involved in the development of NV.
This study had an age- and sex-matched and case-control design involving the retrospective review of medical records. The institutional review board of Seoul National University Hospital (SNUH) approved the study protocol, and the protocol complied with the tenets of the Declaration of Helsinki. The medical records of 1,645 subjects who wore SCLs on daily basis were reviewed.
The study group comprised 31 patients (31 eyes) assigned to a complications group and 93 age- and sex-matched patients assigned to a control group (93 eyes). All had visited the outpatient clinic at Seoul National University Hospital between January 2008 and April 2011. Patients in the complications group had been diagnosed with corneal NV and had worn SCLs for more than a year. The control subjects were selected from among outpatients who visited for correction of refractive errors in a similar time period, who had worn SCLs for more than a year and who did not have NV. Patients were excluded if they had history of using other types of lenses, had used SCLs less than a year or not at all or did not have adequate medical records for analysis.
The NV was assessed using slit-lamp microscopy. Temporary corneal warpage induced by a contact lens was defined as a 2.0-diopter (D) or higher irregularity index within a 3-mm zone on Orbscan topography (Bausch & Lomb, Rochester, NY, USA). Corneal thickness was measured by ultrasound pachymetry (Quantel Medical, Clermont-Ferrand, France) and the base curve radius of the cornea, spherical equivalent and corneal astigmatism were evaluated by automated keratometry (KR-8900; Topcon, Tokyo, Japan). In this study, the best-corrected visual acuity was determined at the first visit.
The demographic data of the two groups were statistically analyzed using Fisher's exact test for comparing proportions and independent Student t-tests for comparison of means. To evaluate lens-fitting related risk factors that affect corneal complications, binomial logistic regression was used to estimate odds ratios (ORs) as a measure of association. ORs are reported as an estimate of relative risk throughout this study. Univariate models were used to determine the individual effect of each measure on the odds of a superficial corneal complication occurring. A multivariate model incorporated all significant factors (p < 0.05) from the univariate models. The statistical software used was SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
First, we looked at whether the demographics of the 2 groups were different from each other (Table 1). The mean age of all subjects was 29.1 ± 8.8 years and most were women (98 / 124, 72.1%). The average duration of contact lens use was 7.55 ± 6.078 years. Other than visual acuity and spherical equivalent, most demographic factors were not significantly different between groups. The low visual acuity observed in the NV group is presumably related to the presence of superficial punctate keratitis or opacity accompanied by NV in some patients. Corneal warpage was found in 19.3% (6 / 31) of the patients in the NV group, but this was not significantly different from the control group. Corneal NV improved in 41.9% (13 / 31) three months after discontinuing lens wear, and six patients (6 / 31, 19.3%) showed complete remission 4.67 ± 3.88 months after giving up lenses. Other factors, such as SCL oxygen transmissibility, lens exchange schedules and care solutions, had no significant effect on the NV group compared to controls.
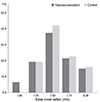
Next, we assessed the cut-off value of parameters for contact-lens fitting as risk factors. Frequency distribution graphs had the potential to provide an overview of cut-off values before univariate and multivariate analysis. The representative complications, frequency distribution of spherical equivalent, astigmatism, and the base curve radius of the cornea for SCL users who did and did not develop NV are exhibited in Figs. 1, 2, 3, and 4. The greater proportion of complication group to control group was revealed in above -9 D of spherical equivalent. The degree of astigmatism in the complications group was also higher in proportion to control group, especially greater than -2 D. The complications group also exhibited steeper corneas, in which the base curve radius was 7.40 mm less than that of the control group.
Finally, we compared both groups employing univariate and multivariate models using the ORs for risk factors selected from the frequency distribution graphs (Table 2). In the univariate model, myopia (>-9 D) demonstrated a significant association with NV (OR, 2.232; 95% confidence interval [CI], 1.602 to 3.105). Patients with high astigmatism (≥ 2 D) had a tendency to develop NV (OR, 2.717; 95% CI, 1.141 to 6.451). People with a steep base curve radius (<7.5 mm) had a 4.000-fold greater odds (95% CI, 1.661 to 9.804). In the multivariate model, spherical equivalent was revealed to be an important risk factor for NV, but astigmatism and steep cornea were not statistically significant risk factors. Myopic eyes (>-9 D) were more likely to experience a corneal surface complication (OR, 2.057; 95% CI, 1.359 to 3.115), while astigmatism (≥2 D) did not significantly affect the development of NV (OR, 0.915; 95% CI, 0.330 to 2.538). A steep cornea (<7.5 mm) was associated with an OR of 1.745 for NV, but this was not significant (95% CI, 0.647 to 4.717); in contrast, having a flat cornea did not affect the eye surface.
Our data suggested that high myopia, high astigmatism, and steep cornea may be important risk factors in the development of NV in people using hydrogel SCL daily over a long period. There are several potential reasons for the occurrence of ocular surface complications in long-term contact wearers: 1) high myopic lenses can increase the peripheral thickness of SCLs, resulting in a reduction of peripheral oxygen transmissibility; 2) high astigmatic lenses can lead to inadequate alignment of the cornea, potentially causing an increase in peripheral mechanical friction; and 3) inappropriate lens-corneal alignment owing to a too-steep cornea fit with a SCL in which the base curve radius ranges from 8.60 to 8.90 mm could induce mechanical damage by interfering with adequate tear circulation.
Oxygen permeability (Dk) is characterizes a given material, while oxygen transmissibility (Dk/L) indicates the oxygen permeability (Dk) divided by lens thickness (L), and represents the amount of oxygen that passes through a lens and is permitted to reach the cornea. Generally, peripheral Dk/L is lower than central Dk/L in commonly used SCLs, because peripheral thickness is typically greater than central thickness [15,16]. The peripheral thickness of a lens with low Dk/L is involved in limbal hyperemia, which is suggestive of limbal hypoxia [16]. In addition, the peripheral thickness of a myopic lens increases depending on the lens power and diameter. Taken together, high myopic correction with SCLs could cause chronic hypoxia and focal limbal deficiency in long-term users. It is not clear to what extent diopteric correction might be an actual risk factor requiring special attention when prescribing SCLs, which is why we sought to establish cut-off points. Our study suggests that patients who have a prescription for lenses with higher than -9 D should undergo regular check-ups for corneal hypoxia and NV.
Chalmers et al. [6] and Wagner et al. [17] also considered lens power to be one of the risk factors for adverse contact lens-associated events, in agreement our results. Chalmers et al. [6] and Wagner et al. [17] reported that levels of ametropia greater than 5.0 D were associated with the risk of contact lens-related complications. They included both hyperopic and myopic diopter lower than 5.0 D were associated with events during contact-lens wear (OR, 0.75 to 0.8). Meanwhile, we focused on myopic correction alone, because in Korea the population of myopic patients is far larger than the population of hyperopic patients, and we specifically targeted ocular surface complications indicating that chronic hypoxia arises from variable contact-lens events (e.g., allergy, infiltrates, infection, and acute abrasion). This explains why the cut-off value of our study varies from that of other studies; furthermore, our data strongly suggest that contact lens correction of myopia greater than -9 D may be required for chronic limbal hypoxia, resulting in NV with a high OR (OR, 2.057). We also assumed that the eyelids of Koreans were more tensive than that of Caucasians, which could lead to variation in the mechanical effect of SCLs between these two studies. Additionally, their study had a different age distribution (11 to 34 years) and study design (controlled, randomized, prospective clinical trials) from our report; these factors may account for the variation in results seen between the two studies.
There have been no previous reports investigating the correlation between astigmatism and corneal complications. Nevertheless, corneal toricity may increase tight sealing and peripheral clearance, which could interfere with tear circulation in the steep direction and enhance mechanical compression in the flat direction when used with spherical lenses; therefore, it can be presumed that some damage would occur in both directions, potentially resulting in ocular complications.
In addition, a steep base curve radius of the cornea may also contribute to ischemic or mechanical change in corneas when SCLs are used. Oxygen is supplied by both direct oxygen flow through the lens and indirect oxygen dissolved in tears in low oxygen-transmissible SCL users. Considering that the usual base curve radius of SCLs ranges from 8.60 to 8.90 mm, bad corneal-lens alignment due to a steep cornea increases squeezing in the center due to flat fitting, and, subsequently, tear circulation can be disturbed, resulting in both ischemic and mechanical damage.
Our study had several limitations. A case-controlled study can have some intrinsic biases like ascertainment bias, Berkson's bias (admission rate bias) and referral bias. However, ascertainment bias might not be a problem in this study because all cases with uncertainty of lens wear duration or of the kind of the lens (soft/hard) were excluded. We also did not include any admitted patients, which prevented Berkson's bias [18]. Finally, SNUH is a tertiary hospital in Korea; therefore, patients at SNUH may present with more severe conditions compared to the general population, leading to a referral bias. Although we could not fully address the referral bias, this results provide practical clinical information for us.
Nevertheless, this appears to be the first report to highlight the importance of contact-lens fitting parameters as biometric risk factors in ocular surface complications following extended periods of daily wear of SCLs.
In conclusion, our study indicated that high myopia, high astigmatism, and a steep cornea (<7.5 mm) might be biometric risk factors associated with the development of corneal surface complications in SCL wearers. Close follow-up for ocular surface complications is recommended when prescribing contact lenses for patients with myopia greater than 9 D, astigmatism greater than 2.0 D, or a base curve radius <7.5 mm.
Figures and Tables
Fig. 1
Representative images of complications. (A) Corneal neovascularization at superior cornea. (B) Severe corneal warpage on Orbscan topography.
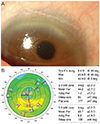
Fig. 2
The frequency distribution of spherical equivalents among soft contact lens wearers (control group vs. corneal neovascularization group).

Fig. 3
The frequency distribution of astigmatism among soft contact lens wearers between (control group vs. corneal neovascularization group).
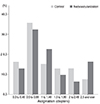
Fig. 4
The frequency distribution of base curve radius of cornea among soft contact lens wearers (control group vs. corneal neovascularization group).
References
1. Robertson DM, Petroll WM, Jester JV, Cavanagh HD. The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: an overview. Eye Contact Lens. 2007; 33:394–398.
2. Chalmers RL, Keay L, Long B, et al. Risk factors for contact lens complications in US clinical practices. Optom Vis Sci. 2010; 87:725–735.
3. Jones LW, Jones DA. Non-inflammatory corneal complications of contact lens wear. Cont Lens Anterior Eye. 2001; 24:73–79.
4. Markoulli M, Papas E, Cole N, Holden B. Corneal erosions in contact lens wear. Cont Lens Anterior Eye. 2012; 35:2–8.
5. Teo L, Lim L, Tan DT, et al. A survey of contact lens complications in Singapore. Eye Contact Lens. 2011; 37:16–19.
6. Chalmers RL, Wagner H, Mitchell GL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci. 2011; 52:6690–6696.
7. Dart JK, Radford CF, Minassian D, et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008; 115:1647–1654.
8. Nichols JJ, Sinnott LT. Tear film, contact lens, and patient factors associated with corneal staining. Invest Ophthalmol Vis Sci. 2011; 52:1127–1137.
9. Ozkan J, Mandathara P, Krishna P, et al. Risk factors for corneal inflammatory and mechanical events with extended wear silicone hydrogel contact lenses. Optom Vis Sci. 2010; 87:847–853.
10. Ramamoorthy P, Sinnott LT, Nichols JJ. Treatment, material, care, and patient-related factors in contact lens-related dry eye. Optom Vis Sci. 2008; 85:764–772.
11. Richdale K, Sinnott LT, Skadahl E, Nichols JJ. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea. 2007; 26:168–174.
12. Stapleton F, Edwards K, Keay L, et al. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology. 2012; 119:1516–1521.
13. Martin R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom. 2007; 90:26–30.
14. Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011; 30:18–23.
15. Morgan PB, Brennan NA, Maldonado-Codina C, et al. Central and peripheral oxygen transmissibility thresholds to avoid corneal swelling during open eye soft contact lens wear. J Biomed Mater Res B Appl Biomater. 2010; 92:361–365.
16. Papas E. On the relationship between soft contact lens oxygen transmissibility and induced limbal hyperaemia. Exp Eye Res. 1998; 67:125–131.
17. Wagner H, Chalmers RL, Mitchell GL, et al. Risk factors for interruption to soft contact lens wear in children and young adults. Optom Vis Sci. 2011; 88:973–980.
18. Sutton-Tyrrell K. Assessing bias in case-control studies. Proper selection of cases and controls. Stroke. 1991; 22:938–942.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download