Abstract
Purpose
To examine the effects of panretinal photocoagulation (PRP) using a pattern scanning laser (PASCAL) system on the retinal nerve fiber layer (RNFL) thickness in patients with diabetic retinopathy.
Methods
This retrospective study included 105 eyes with diabetic retinopathy, which consisted of three groups: the PASCAL group that underwent PRP with the PASCAL method (33 eyes), the conventional group that underwent conventional PRP treatment (34 eyes), and the control group that did not receive PRP (38 eyes). The peripapillary RNFL thickness was measured by optical coherence tomography before, six months, and one year after PRP to evaluate the changes in peripapillary RNFL.
Results
The RNFL thickness in the PASCAL group did not show a significant difference after six months (average 3.7 times, p = 0.15) or one year after the PRP (average 3.7 times, p = 0.086), whereas that in the conventional group decreased significantly after six months (average 3.4 times, p < 0.001) and one year after PRP (average 3.4 times, p < 0.001).
Panretinal photocoagulation (PRP) has become the gold standard for the treatment of diabetic retinopathy [1,2,3,4,5,6]. The Diabetic Retinopathy Study, the Early Treatment Diabetic Retinopathy Study, and other studies [1,2] have demonstrated the effectiveness of laser treatment in the control of these diseases. PRP has been recommended in eyes with severe diabetic retinopathy, including those with severe non-proliferative diabetic retinopathy or non-high-risk proliferative retinopathy [1]. However, conventional PRP treatment has raised concerns regarding the decrease in peripapillary retinal nerve fiber layer (RNFL) thickness due to thermal damage [1]. Hsu and Chung [7] investigated changes in the RNFL after PRP in diabetic eyes and suggested that the decrease in RNFL thickness may be due to direct damage from the glycosylation end products of diabetes and thermal damage from photocoagulation. Another study by Lim et al. [8] reported that the RNFLs in the PRP group were thinner than those in the diabetic group that did not undergo PRP, which implied that PRP might cause the thinning of RNFL beyond the effect of diabetes alone [1,2,3].
A new alternative laser method, the pattern scanning laser (PASCAL; Opti-Media Corp., Santa Clara, CA, USA) system, is a semi-automated photocoagulator. The PASCAL system can reduce total pulse energy effectively by lessening the laser shot duration [9,10,11]. Thus, the use of thermal energy is less than that in conventional PRP [9]. We hypothesized that the PASCAL system could protect against a decrease in peripapillary RNFL thickness due to thermal damage. However, there have been no studies to date on whether PASCAL influences RNFL thickness.
The present study will investigate changes in RNFL thickness after PASCAL photocoagulation in diabetic patients to improve our understanding of whether PASCAL photocoagulation might alter RNFL thickness.
The study design followed the tenets of the Declaration of Helsinki for biomedical research and was approved by the institutional review board of the Catholic University of Korea.
This retrospective study included 105 eyes from 105 patients with diabetic retinopathy. They were divided into three groups: a conventional treatment group who had received PRP by the conventional method, a PASCAL treatment group who had received PRP by the PASCAL system, and a control group who did not receive any PRP from January 2008 to October 2011.
Patients in the conventional PRP group and the PASCAL PRP group had reached the stage of severe non-proliferative diabetic retinopathy or worse. All patients in the control group had at most moderate non-proliferative diabetic retinopathy without receiving laser treatment. Subjects were excluded if they had significant ocular disease other than diabetic retinopathy, a history of glaucoma, or suspected glaucoma.
All sessions of the conventional PRP group were conducted by one ophthalmologist (DHJ) with a Novus Omni laser system (Coherent, Santa Clara, CA, USA). All sessions of the PASCAL PRP group were conducted by the same ophthalmologist (DHJ) with a PASCAL photocoagulator, which is a 532-nm double-frequency Nd:YAG laser. For both groups, the 2 × 2, 3 × 3, 4 × 4, and 5 × 5 arrays were most commonly used. All burns were applied one burn width apart. All treatments were performed with a Mainster PRP 165 contact lens (Ocular Instruments, Bellevue, WA, USA), which had a burn size magnification power of 1.96×.
Peripapillary RNFL measurements were obtained using optical coherence tomography (Stratus OCT 3, Carl Zeiss Meditech, Dublin, CA, USA). Through a dilated pupil which was more than 6 mm in size, a 3.4-mm diameter ring was centered around the optic nerve head and 768 A-scans were acquired using the fast RNFL thickness protocol. The following regions were assessed: temporal (316° to 45°), superior (46° to 135°), nasal (136° to 225°), and inferior (226° to 315°). In clock-hour positions, 2 to 4 o'clock was considered temporal; 5 to 7 o'clock, inferior; 8 to 10 o'clock, nasal; and 11 to 1 o'clock, superior. The average thickness (360°) was also measured. In the conventional PRP group and the PASCAL PRP group, the peripapillary RNFL thickness was measured before the PRP, six months after the PRP, and one year after the PRP. In the control group, the RNFL thickness was measured at baseline, six months later, and one year later. We collected the patients' age, sex, duration of diabetes, visual acuity, and intraocular pressure. Statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A series of repeated-measures paired t-tests was used to examine the interval changes in the RNFL thicknesses as time passed in each group.
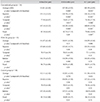
In total, 34 eyes of 34 diabetic patients who underwent conventional PRP (the conventional PRP group), 33 eyes of 33 diabetic patients who underwent PRP by the PASCAL system (the PASCAL PRP group), and 38 eyes of 38 subjects with diabetes who did not receive PRP (the control group) were included in this study. Patient demographic data showed no statistically significant differences in age, sex, visual acuity, or intraocular pressure between the three groups (Table 1).
There were no significant differences in the superior, temporal, inferior, nasal, or average RNFL thicknesses between the conventional group, the PASCAL group, and the control group at the baseline. Six months after the PRP procedures, there were no significant differences in RNFL thicknesses in the PASCAL group or the control group, but there was a significant decrease in the RNFL thickness in the conventional PRP group (3.70 ± 4.24 µm, p < 0.001). One year after the PRP procedures, there were still no significant differences in RNFL thickness in the PASCAL group or the control group, whilst the RNFL thickness decreased significantly in the conventional PRP group (5.42 ± 3.69 µm, p < 0.001). There was no significant difference in the RNFL thicknesses at six months and one year after the PRP procedure in the conventional PRP group (Table 2).
The purpose of this study was to evaluate the effect of the PASCAL system on RNFL damage in patients with diabetic retinopathy. In our study, the PASCAL PRP group showed no significant difference in RNFL thickness after six months or after one year as compared with the thickness at baseline. However, RNFL thickness was significantly decreased after six months and after one year in the conventional PRP group.
A typical PRP session, which remains the treatment of choice for severe diabetic retinopathy, consists of laser burns with diameters of 100 to 200 µm at the retina with pulse durations ranging from 50 to 300 ms [1,2]. With these standard parameters, the energy is absorbed by the retinal pigment epithelium and produces thermal damage to the outer retina. However, the amount of heat generated at this level affects the neighboring tissue, significantly expanding the area of original burn damage [1,2,3,10]. If the laser intensity is too strong and destroys the entire retinal layer, it will cause a sequential decrease in RNFL thickness [1,2,6].
RNFL thickness has been proven to decrease after PRP. Hsu and Chung [7] documented that the superior/temporal and inferior/temporal ratios were more reliable in evaluating nerve fiber analyzer data in patients with diabetic retinopathy after PRP. Lim et al. [8] reported similar results while studying the effects of diabetic retinopathy and PRP on RNFL and optic nerve appearance. They argued that diabetic eyes that have been treated with PRP have thinner RNFLs than nondiabetic eyes do. The optic nerves in eyes treated with PRP are more likely to be graded as abnormal, but their appearance is not necessarily glaucomatous and this may be related to thinning of the RNFL. Recently, Kim and Cho [12] documented that the decrease in the RNFL thickness of a PRP group was not statistically significant compared to the control group. However, they suggested RNFL was significantly vulnerable to laser damage at higher blood HbA1c levels.
The PASCAL (Opti-Media Corp.) system is a semi-automated photocoagulator that allows the placement of 1 to 56 burns per activation of the foot-pedal, in pre-configured patterns. The system uses short pulse durations of 10 to 30 ms accompanied by significantly greater power. In traditional photocoagulation, with pulse durations of 100 to 200 ms, the laser burn size is highly dependent on the laser power and pigment distribution, and can exceed the beam diameter by a factor of up to three. With the shortened pulses (10 to 30 ms), the thermal diffusion is decreased, limiting the damage to surrounding tissue, and the physical mechanism causing the lesions is not only thermal but also mechanical, due in part to the formation of microbubbles at the melanosomes [13,14]. As the laser pulse duration and associated duration of hyperthermia decrease, the temperature required to achieve the same extent of coagulation has to increase, so 1.5 to 3 times the normal power is required to produce clinically useful burns [15,16,17]. However, due to the fact that the pulse energy (power × duration) is still three times lower than that in conventional photocoagulation, the reduced thermal damage to RNFL can decrease RNFL loss.
The clinical safety of the PASCAL system had been proved by Muqit et al. [18]. They reported that the clinical efficacy of the PASCAL laser using 10- to 30-ms pulse durations was comparable to the conventional standard protocols used for the treatment of vascular retinal disorders, and that higher power with 10- to 30-ms pulse durations might be safely and effectively used in clinical practice. Sheth et al. [17] performed 1242 PASCAL laser procedures in 752 eyes of Caucasians and documented that the consequent reduction in heat dissipation, especially in the lateral direction, can allow one to use relatively larger spot sizes and apply more closely spaced burns without incurring significant side effects. Although the laser burn grade is influenced by several factors, a major factor is the total energy of the laser. The total energy of the PASCAL system is one-third that of conventional PRP, which suggests that the PASCAL system can be safer than conventional PRP. These results suggest that the PASCAL system can produce effective burns with less thermal damage on the RNFL, whereas conventional PRP increases the risk of thermal damage to the RNFL.
This study has several limitations. The retrospective, non-randomized study design has the possibility of selection bias. Specifically, most of the patients received conventional PRP in an early period before the introduction of PASCAL. Another limitation is the small number of cases, which weakens the statistical power to distinguish the differences among the groups. Future studies should examine prospective randomized results to verify our results.
In conclusion, the PASCAL system uses less energy for effective photocoagulation burns and protects against RNFL damage by producing less thermal damage. Therefore, the PASCAL system can be a good alternative laser method for preserving retinal function by protecting RNFL thickness.
Figures and Tables
References
1. Ferris FL 3rd. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic retinopathy. JAMA. 1991; 266:1263–1265.
2. Central Vein Occlusion Study Group. Central vein occlusion study of photocoagulation therapy.
Baseline findings. Online J Curr Clin Trials. 1993; Doc No 95.
3. Lonneville YH, Ozdek SC, Onol M, et al. The effect of blood glucose regulation on retinal nerve fiber layer thickness in diabetic patients. Ophthalmologica. 2003; 217:347–350.
4. Ai E. Current management of diabetic retinopathy. West J Med. 1992; 157:67–70.
5. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995; 102:1434–1444.
6. Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96:211–216.
7. Hsu SY, Chung CP. Evaluation of retinal nerve fiber layer thickness in diabetic retinopathy after panretinal photocoagulation. Kaohsiung J Med Sci. 2002; 18:397–400.
8. Lim MC, Tanimoto SA, Furlani BA, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol. 2009; 127:857–862.
9. Ozdek S, Lonneville YH, Onol M, et al. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye (Lond). 2002; 16:761–765.
10. Jain A, Blumenkranz MS, Paulus Y, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol. 2008; 126:78–85.
11. Sanghvi C, McLauchlan R, Delgado C, et al. Initial experience with the Pascal photocoagulator: a pilot study of 75 procedures. Br J Ophthalmol. 2008; 92:1061–1064.
12. Kim HY, Cho HK. Peripapillary retinal nerve fiber layer thickness change after panretinal photocoagulation in patients with diabetic retinopathy. Korean J Ophthalmol. 2009; 23:23–26.
13. Blumenkranz MS, Yellachich D, Andersen DE, et al. Semiautomated patterned scanning laser for retinal photocoagulation. Retina. 2006; 26:370–376.
14. Tso MO, Wallow IH, Elgin S. Experimental photocoagulation of the human retina. I. Correlation of physical, clinical, and pathologic data. Arch Ophthalmol. 1977; 95:1035–1040.
15. Sugimoto M, Sasoh M, Ido M, et al. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005; 219:379–385.
16. Al-Hussainy S, Dodson PM, Gibson JM. Pain response and follow-up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye (Lond). 2008; 22:96–99.
17. Sheth S, Lanzetta P, Veritti D, et al. Experience with the Pascal photocoagulator: an analysis of over 1,200 laser procedures with regard to parameter refinement. Indian J Ophthalmol. 2011; 59:87–91.
18. Muqit MM, Sanghvi C, McLauchlan R, et al. Study of clinical applications and safety for Pascal laser photocoagulation in retinal vascular disorders. Acta Ophthalmol. 2012; 90:155–161.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download