Abstract
Giant cell arteritis (GCA) is a rare disease among Asians. Arteritic anterior ischemic optic neuropathy, which accompanies GCA, has not yet been reported in Koreans. Diagnosis of GCA is difficult if typical symptoms other than visual loss are absent. Here, we report a case of an 83-year-old Korean woman presenting with sudden visual loss in both eyes (oculus uterque, OU). Her visual acuities included perception of light in the right eye (oculus dexter, OD) and perception of hand motion in the left eye (oculus sinister, OS). The results of the Hardy-Rand-Rittler test and Ishihara test showed total dyschromatopsia OU. The Goldmann perimetry test revealed a total field defect OD and paracentral island OS. Fundus examination revealed chalky-white disc swelling OU. Other systemic symptoms and signs were unremarkable. The erythrocyte sedimentation rate, C-reactive protein and platelet count were highly elevated. Temporal artery biopsy revealed multiple lymphocytes and multinucleated giant cells in the arterial media layer. To our knowledge, this is the first report of GCA in a Korean that has been confirmed with temporal artery biopsy. In conclusion, silent GCA can occur in Koreans, and hence, elderly patients presenting with chalky-white disc swelling, and corresponding laboratory findings must be evaluated for GCA.
Giant cell arteritis (GCA), or temporal arteritis, is a vasculitis of medium-to-large-sized arteries that is confirmed with temporal artery biopsy. In GCA, the arterial walls are infiltrated with inflammatory cells such as lymphocytes, epithelioid histiocytes (giant cells), macrophages and fibroblasts. Ophthalmic involvement can occur in up to 50% to 70% of the GCA patients, and this represents an ocular emergency [1,2]. Arteritic anterior ischemic optic neuropathy (AAION) is the most common type of ophthalmic involvement in GCA and can cause permanent visual loss. Therefore, prompt diagnosis and treatment with a high dose of steroids is essential for these patients.
Patients with GCA typically present with headache, jaw claudication, fever, weight loss, myalgia, arthralgia, or malaise [1]. Conversely, patients with silent GCA, first described by Simmons and Cogan [3], present with sudden visual loss without systemic symptoms and signs. Thus, due to the lack of symptoms, diagnosis and treatment of silent GCA may be considerably delayed when compared to typical GCA [4]. An elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level and platelet count can serve as sensitive indicators in the diagnosis of silent GCA [2]. There have only been a few reports of silent GCA patients in Asia [5,6], and no reports on patients with GCA-accompanied AAION in Korea. Here, we report a case of silent GCA in an elderly Korean woman, as confirmed by temporal artery biopsy.
An 83-year-old woman presented with sudden visual loss that had developed in both eyes (oculus uterque, OU) the previous day. Her past medical history was unremarkable. She did not complain of any associated headache, scalp tenderness, jaw claudication or constitutional symptoms such as weight loss, fever, malaise or sweats. Her visual acuities included perception of light in the right eye (oculus dexter, OD) and perception of hand motion in the left eye (oculus sinister, OS). The anterior segment examination revealed advanced nuclear sclerosis OU. A relative afferent pupillary defect OD was detected. Fundus examination revealed mild retinal arterial narrowing and chalky-white disc swelling OU (Fig. 1). The results of the Hardy-Rand-Rittler test and Ishihara test showed total dyschromatopsia OU. The Goldmann perimetry test revealed a total field defect OD and paracentral island OS. Although electroretinography findings were within normal limits, visual evoked potentials showed delayed P100 latency OU. Cerebrospinal fluid tapping revealed normal intracranial pressure and cell counts. Brain magnetic resonance imaging scans and angiography results showed diffuse bilateral stenosis of vertebral arteries and external carotid arteries without significant intracranial vessels stenosis. The CRP level, ESR and platelet count were elevated and measured to be 5 mg/dL (upper normal limit, 0.5 mg/dL), 55 mm/h (upper normal limit, 20 mm/h), and 510 K/µL (upper normal limit, 400 K/µL), respectively.
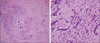
Following a presumptive diagnosis of silent GCA-associated AAION OU, the patient was hospitalized and treated with intravenous 250 mg methylprednisolone every 6 hours for 3 days. Biopsy of the left temporal artery was performed, and 3 cm of the temporal artery was acquired. Lymphocytes, epithelioid histiocytes, and multinucleated giant cells had diffusely infiltrated into the entire vessel wall, especially in the arterial wall media (Fig. 2). GCA was confirmed, and a high dose of steroid therapy was maintained.
After 7 days, the patient's visual acuity OS improved slightly to being able to count fingers. Disc swelling OU had decreased. The CRP level and ESR decreased to 1.07 mg/dL and 30 mm/h, respectively. Oral prednisolone therapy was slowly tapered down from 60 mg per day, and steroid treatment was maintained with deflazacort 30 mg per day. However, after 4 months, the patient's visual acuities deteriorated to no light perception OD and light perception OS.
GCA predominantly affects elderly Caucasian females. GCA should be strongly suspected when patients greater than 50 years of age present with headaches. The incidence of GCA in Scandivian countries and North America ranges between 6.9 and 32.8 per 100,000 [7]. However, the occurrence of GCA is rare in African Americans, Hispanics, and Asians [8-10]. There have only been a few reports of GCA among Asians [5,6,11-15]. In Japan, a nationwide GCA survey revealed an extremely low prevalence of 1.47 per 100,000 population, which is approximately 1 / 140 of that reported in the US [10]. Pereira et al. [16] reported that GCA was seen 20 times less frequently in Asians than in Caucasians. Chaudhry et al. [17] stated that, for over a period of 22 years, only 7 patients were diagnosed with GCA by temporal artery biopsy in a tertiary medical center in Saudi Arabia.
The incidence of GCA in Asians was far lower than that in Caucasians; however, the incidence is now increasing in the Asian population. After 36 years of no reported cases of GCA, in 2010, Aui-Aree et al. [6] reported 4 GCA cases in Thailand. Cullen et al. [5] noted that of the 7 biopsy-confirmed GCA cases reported over the past 10 years in Singapore, 3 were reported in 2009. This trend may be associated with an increase in the maximum life span of the Asian population [7]. Suspected diagnosis of GCA by rheumatologists and ophthalmologists, along with extensive laboratory tests, may be other important factors. However, a nation-wide epidemiologic study would be needed to clarify the association of life span and GCA incidence in Asians.
In Korea, only a few biopsy-confirmed GCA cases have been reported [18,19]; however, there have been no GCA-associated AAION cases so far. To our knowledge, this is the first biopsy-confirmed report of GCA-associated AAION in Korea. Our patient was diagnosed with silent GCA, and the clinical features overlapped with non-arteritic AION. When Asian patients aged >50 years present with acute visual loss and disc swelling and no other symptom, non-arteritic AION accounts for more than 90% of these cases. In such circumstances, laboratory parameters, such as ESR, CRP level and platelet count can serve as indicators in the diagnosis of GCA. Hayreh et al. [20] reported that the CRP level has a sensitivity of 100% for GCA, and the combination of CRP level and ESR has a specificity of 97%. In a large population-based cross-sectional study, Walvick et al. [21] documented that the odds of a positive biopsy were 1.5 times greater with an ESR of 47 to 100 mm/h, 5.3 times greater with a CRP of >2.45 mg/dL, and 4.2 times greater with a platelet count of >400,000 µL. The above 3 parameters were elevated in our patient (CRP, 5 mg/dL; ESR, 55 mm/h; and platelet count, 510 K/µL). These test results are known to be normal in non-arteritic AION.
In conclusion, although this disease is rare in Asians, GCA-associated AAION should be considered when an elderly patient presents with sudden visual loss and disc edema. GCA should be suspected and laboratory tests should be performed, even in the absence of typical symptoms.
Figures and Tables
Fig. 1
Fundoscopic findings. Chalky-white disc swellings were found in both eyes (A, right eye; B, left eye), especially in the right eye (A).
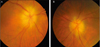
Fig. 2
Histopathology of left temporal arterial biopsy. (A) Diffuse infiltration by multiple inflammatory cells including lymphocytes, macrophages and multinucleated giant cells into all layers of the arterial wall, especially the media (H&E, ×100). (B) A few multinucleated giant cells among the diffusely infiltrated lymphocytes (H&E, ×400).
References
1. Rahman W, Rahman FZ. Giant cell (temporal) arteritis: an overview and update. Surv Ophthalmol. 2005. 50:415–428.
2. Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis: ocular manifestations. Am J Ophthalmol. 1998. 125:521–526.
3. Simmons RJ, Cogan DG. Occult temporal arteritis. Arch Ophthalmol. 1962. 68:8–18.
4. Ezeonyeji AN, Borg FA, Dasgupta B. Delays in recognition and management of giant cell arteritis: results from a retrospective audit. Clin Rheumatol. 2011. 30:259–262.
5. Cullen JF, Chan BM, Wong CF, Chew WC. Giant cell (temporal) arteritis in Singapore: an occult case and the rationale of treatment. Singapore Med J. 2010. 51:73–77.
6. Aui-Aree N, Tungsinmunkong K, Hirunpat S, et al. A variety of atypical manifestations in giant cell arteritis. J Med Assoc Thai. 2010. 93:629–632.
7. Lee JL, Naguwa SM, Cheema GS, Gershwin ME. The geoepidemiology of temporal (giant cell) arteritis. Clin Rev Allergy Immunol. 2008. 35:88–95.
8. Liu NH, LaBree LD, Feldon SE, Rao NA. The epidemiology of giant cell arteritis: a 12-year retrospective study. Ophthalmology. 2001. 108:1145–1149.
9. Smith CA, Fidler WJ, Pinals RS. The epidemiology of giant cell arteritis: report of a ten-year study in Shelby County, Tennessee. Arthritis Rheum. 1983. 26:1214–1219.
10. Kobayashi S, Yano T, Matsumoto Y, et al. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum. 2003. 49:594–598.
11. Cullen JF, Chan CM, Chuah KL. Giant cell arteritis (temporal arteritis, cranial arteritis) and a case from Singapore. Singapore Med J. 2003. 44:306–308.
12. Cheng CK, Lee CC, Huang KH, et al. Giant cell (temporal) arteritis with anterior ischemic optic neuropathy: a biopsy-proven case in Taiwan. J Formos Med Assoc. 2010. 109:550–554.
13. Wang X, Hu Z, Lu W, et al. Giant cell arteritis: a rare disease in Asians. J Clin Rheumatol. 2009. 15:48.
14. Chen CH, Kung SY, Tsai YY, et al. Temporal arteritis. J Chin Med Assoc. 2005. 68:333–335.
15. Kwok AK, Lam DS, Liew CT. Bilateral arteritic central retinal artery occlusion in a Chinese patient. Aust N Z J Ophthalmol. 1998. 26:175–176.
16. Pereira LS, Yoon MK, Hwang TN, et al. Giant cell arteritis in Asians: a comparative study. Br J Ophthalmol. 2011. 95:214–216.
17. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Epidemiology of giant-cell arteritis in an Arab population: a 22-year study. Br J Ophthalmol. 2007. 91:715–718.
18. Kwon CM, Hong YH, Chun KA, et al. A case of silent giant cell arteritis involving the entire aorta, carotid artery and brachial artery screened by integrated PET/CT. Clin Rheumatol. 2007. 26:1959–1962.
19. Kim KH, Yang WI, Choi IJ. Giant cell arteritis of the breast: a case report. Yonsei Med J. 1990. 31:80–84.
20. Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997. 123:285–296.
21. Walvick MD, Walvick MP. Giant cell arteritis: laboratory predictors of a positive temporal artery biopsy. Ophthalmology. 2011. 118:1201–1204.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download