Abstract
Purpose
To assess the results and long-term prognosis of evisceration with primary porous implant placement in patients with endophthalmitis.
Methods
A retrospective study was conducted to review the files of 27 patients (29 eyes) with endophthalmitis who underwent evisceration with primary porous implant placement from January 1997 to December 2007 at St. Mary's Hospital and Kangnam St. Mary's Hospital. The mean follow-up period was 12.24 months (range, 3 to 89 months) and the mean age of the patients was 63.6 years (range, 33 to 89 years).
Results
During the surgical procedure, primary implant placement was successfully completed, and any postoperative infection or inflammation rapidly resolved in all 27 patients (29 eyes). One of two porous implant materials was used. Hydroxyapatite was inserted in 14 eyes and Medpor was inserted in 15 eyes. Delayed implant exposure was noted in 1 eye, which was treated by inserting a hydroxyapatite implant 18 months after the first surgery. This was well treated by a preserved scleral graft. Implant infection was noted in 1 other eye at 20 days after the first surgery. All other minor complications healed without sequelae.
Conclusions
Evisceration with primary porous implant placement as the treatment for recalcitrant endophthalmitis resulted in rapid resolution of any infection and inflammation. Implant exposure and infection occurred in only 2 eyes, and these problems were well treated without long-term sequelae. Therefore, evisceration with primary porous implant placement is a treatment option for patients with endophthalmitis.
Endophthalmitis can be caused by many events, including intraocular surgery, trauma, corneal ulcer, or systemic infection. The traditional approach for the management of fulminant endophthalmitis that is refractory to other forms of treatment is to perform an evisceration or enucleation [1,2].
With the development of antibiotics, evisceration in the setting of endophthalmitis is preferred to enucleation because evisceration is thought to have less risk of postoperative meningitis or encephalitis. But in most cases, the use of orbital implantation has been restricted to a silicone ball or polymethyl methacrylate (PMMA) [3].
Porous orbital implants are now commonly used after encucleation and evisceration and as secondary orbital implants. The advantage of porous orbital implants is that the resultant fibrous ingrowth may reduce the risk of extrusion, and small exposures may heal spontaneously. Porous orbital implants also enhance cosmesis and improve the motility of the artificial eye, and so the use of porous orbital implants has increased [4,5].
Yet, there exists the belief that primary implantation of porous implants at the time of evisceration in patients with endophthalmitis carries a high risk of complications such as exposure, extrusion and dissemination of infection. On the basis of this premise, primary porous orbital implantation has not been advocated in the setting of refractory endophthalmitis. Available implant materials have been limited to a silicone ball or PMMA, or patients undergo a two-stage operation (first-stage evisceration followed by delayed second-stage porous orbital implant insertion).
Tawfik and Budin [6] recently reported no complications when 16 porous orbital implants were used in a total of 66 patients with endophthalmitis. However, the general utility of primary porous implant placement at the time of evisceration is not well established with regard to possible infection. The purpose of this study is to evaluate the results of this treatment approach for patients with endophthalmitis.
We reviewed the records of 27 patients (29 eyes) who underwent evisceration with primary porous implant placement by a single surgeon for the management of fulminant endophthalmitis between 1997 and 2007 at St. Mary's Hospital and Kangnam St. Mary's Hospital. The majority of these patients were aggressively treated with topical, intracameral and/or intravenous antibiotics before evisceration. The patients were generally referred when these measures failed to control the infection or the structural integrity of the blind eye was severely compromised. All patients had signs of persistent ocular inflammation. The charts were reviewed for predisposing conditions such as age, the date of surgery, gender, prior ocular surgery and the surgical procedure and technique. The type and size of the orbital implants were also evaluated. In order to assess the organisms that caused the endophthalmitis, the intraocular contents removed during surgery were cultured and the sensitivity of the bacteria was tested. Biopsy was also performed to ascertain that a malignant neoplasm was not misconstrued as endophthalmitis. Finally, the complications that remained, such as infection, inflammation, and exposure or extrusion of the porous orbital implant material, were evaluated. The 'fit' of the postoperative prosthesis and the patients' satisfaction were examined.
For the eviscerations, a 360° peritomy was performed and the sclera was incised just posterior to the surgical limbus. An evisceration spoon or periosteal elevator was used to separate the uvea from the sclera and to deliver the intraocular contents. The interior of the scleral shell was scraped or cleaned with gauze to remove all visible remnants of uveal tissue. In addition, the surgeon routinely used dehydrated (absolute) alcohol to remove residual pigment from the scleral wall. Anterior relaxing incisions that measured approximately 1 cm were made medially and laterally, avoiding the medial and lateral rectus muscles. The posterior sclera was also opened around the optic nerve with a combination of sharp and blunt dissection. A porous implant that was infiltrated with antibiotic solution was placed in the scleral shell, and this was closed in layers with 5-0 Vicryl sutures. The overlying Tenon's capsule and conjunctiva were closed with 6-0 Vicryl suture. A conformer and antibiotic ointment were placed within the fornices and the procedure was completed.
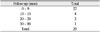
The medical records of 27 patients (29 eyes) who underwent evisceration for endophthalmitis with primary porous orbital implant placement at St. Mary's Hospital and Kangnam St. Mary's Hospital were reviewed (Table 1). The ages of the patients ranged from 33 to 89 years (mean age, 63.6 years). There were 17 females and 12 males (Table 2). The mean follow-up period was 12.2 months (range, 3 to 89 months) (Table 3).
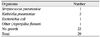
The culture and sensitivity results were available for 29 patients. The result was no growth in 23 patients, presumably because of the heavy administration of topical and systemic antibiotics prior to surgery. The culture results were positive for Streptococcus pneumoniae in 1 patient, Klebsiella pneumoniae in 3 patients and Escherichia coli in 1 patient; 1 culture grew Aspergillus flavum (Table 4). Histopathologic evaluation of the intraocular contents was routinely performed for all patients to rule out any incidental finding of malignancy, but none was found.
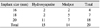
Fifteen patients received hydroxyapatite implants, and 14 patients received Medpor implants. Among the patients who received hydroxyapatite implants, 2 patients received a 16 mm implant, 2 patients received an 18 mm implant and 11 patients received a 20 mm implant. Among those who received Medpor implants, 2 patients received 16 mm implants and 5 patients received 18 mm implants. The remaining 7 patients received a 20 mm Medpor implant (Table 5).
After surgery, implant exposure (13×12 mm) was noted in 1 patient at 16 months after surgery and this was successfully treated with a preserved scleral graft. Suppurative discharge and odor were noted in 1 patient at 20 days after surgery; the abscess was evacuated and the orbital implant was changed to a silicone ball. Other complications included 1 patient in whom marked conjunctival wound dehiscence developed during the early postoperative period (20 days). This conjunctival defect took months to re-epithelialize under conservative treatment without implant exposure (Table 6).
All the patients were successfully fitted with an ocular prosthesis.
In the case of recalcitrant endophthalmitis, evisceration may be preferable to enucleation to reduce the potential risk of intracerebral infectious spread. Evisceration offers many advantages over enucleation, including improved postoperative fornices and implant motility, easier prosthetic fitting and generally improved cosmesis [1,6,7]. Evisceration is considered a safe procedure that has therefore recently gained more interest and acceptance.
Another important consideration after eye removal is selection of an orbital implant. Polyethylene is a high-density, straight-chain hydrocarbon that is formed by polymerization of ethylene molecules under high temperature and pressure. The porous polyethylene orbital implant is manufactured by heating and compaction of polyethylene granules into spherical shapes of different sizes. This material is nontoxic, nonallergenic and highly biocompatible. It is not brittle, thus allowing suturing of muscles directly to it and more importantly, its porous matrix facilitates ingrowth of the host's fibrovascular tissue [8]. This should reduce the risk of migration, exposure and extrusion and minimize the risk of infection. If signs of infection are found, systemic antibiotics work well to treat them [9-11]. The porous orbital implants are relatively more lightweight than a silicone ball or PMMA, which theoretically reduces the pressure over the lower eyelid. Furthermore, if it is needed, this orbital implant is ideally suited for a second operation using peg system or motility coupling post, and it helps to increase movement of the prosthetic eyeball by keeping the extraocular muscles [5,6].
Primary insertion of porous implants after evisceration has been limited to noninfectious situations. This is because a belief exists that primary implantation of porous material at the time of evisceration in the setting of endophthalmitis carries a high risk of complications such as exposure, extrusion and dissemination of infection [11,12]. As a result, the use of orbital implantation has been restricted to a silicone ball or PMMA, or patients undergo a two-stage operation (first-stage evisceration followed by delayed second-stage porous orbital implant insertion) [3,13].
In recent studies, Dresner and Karesh [7] reported 1 porous polyethylene exposure in a series of 11 patients with Pseudomonas aeruginosa endophthalmitis. Tawfik and Budin [6] also reported no complications, including exposure or infection, when 16 porous orbital implants were used for a total 66 patients with endophthalmitis. That study shows the possibility of successful primary porous orbital implant placement in patients with endophthalmitis.
Our results are compatible with those of Dresner and Karesh [7] and Tawfik and Budin [6]. The patients that needed to undergo surgery again due to complications were limited to 2 eyes (6.9%) among 29 eyes that had evisceration with primary porous implant placement because of endophthalmitis. According to Remullar et al. [4], porous orbital implants can be easily exposed when the blood vessels of the implants are not well formed. Without this fibrovascular ingrowth at the anterior surface of the implant, the roughened surface of the implant may lead to conjunctival wound dehiscence and attrition to the sclera and progressive anterior implant migration and eventual exposure, and this can be accompanied by other complications such as infection [9,11].
However, primary porous orbital implant placement with evisceration in patients with endophthalmitis is preferred because it can reduce the patients' concerns about needing additional surgery and it can increase their satisfaction as related to cosmesis. With standard evisceration, it is usually not possible to place anything larger than an 18 mm sphere. Several modifications to the procedure have been reported to enable the placement of larger implants within the scleral cavity. Most of these techniques include cutting the sclera anteroposteriorly with or without separating the optic nerve from the scleral flaps. With this technique, we were able to acquire enough cavity space to insert the implant. After identifying the size of the cavity, we used 20 mm implants in our patients. Possibly, these incisions created a route for fibrovascular ingrowth in the porous orbital implant and thus prevented implant exposure.
With regard to bacterial culture from the surgical site, cultures from 23 out of 29 eyes did not grow organisms. The reason for this is that the patients with endophthalmitis received systemic antibiotics and topical antibiotics before surgery. There was no case in which a patient who received artificial eyes complained about orbital deformities or asked for reconstructive surgery after the initial surgery.
Primary porous implant placement after evisceration can be possible if the surgery is performed selectively. Once an infectious scleritis spreads in the adjacent sclera, medical management alone is usually not effective. It is believed that antibiotics do not adequately penetrate the nearly avascular sclera. Thus, evisceration with primary porous orbital implants may be the procedure of choice in the setting of severe endophthalmitis that is not accompanied by scleral abscesses and perforations.
Moreover, none of our cases showed gross purulent discharge within the orbit. Our approach in the setting of refractory endophthalmitis is to perform an evisceration with a primary porous orbital implant and thoroughly examine the socket for signs of gross contiguous infection. If there is no evidence of gross purulent orbital infection, then we insert a primary porous orbital implant at that time.
In summary, we present a case series of porous orbital implants after evisceration, along with the follow-up results. Based on these results, primary porous implantation after evisceration in patients with endophthalmitis can be tolerated and has a relatively low incidence rate of complications.
The authors note that the results of this study are only a reflection of the particular surgical methods used and should not be used in all cases. In other words, the 1 patient who needed a reoperation due to persistence of infection should not be overlooked. It means the method should be properly chosen according to the patient's condition. It is possible only when a relatively healthy sclera is available because the compromised structural integrity of the sclera may not adequately support an orbital implant.
The results of this study present evisceration with primary porous implants as a new possibility for treatment of patients with endophthalmitis. The proper selection of patients remains mainly a matter of the surgeon's decision, and more research is required to compare outcomes. In conclusion, the results of our study suggest that evisceration with primary porous orbital implant placement for endophthalmitis is an available treatment strategy that carries an acceptably low risk of complications.
Figures and Tables
References
1. O'Donnell BA, Kersten R, McNab A, et al. Enucleation versus evisceration. Clin Experiment Ophthalmol. 2005. 33:5–9.
2. Viswanathan P, Sagoo MS, Olver JM. UK national survey of enucleation, evisceration and orbital implant trends. Br J Ophthalmol. 2007. 91:616–619.
3. Abel AD, Meyer DR. Enucleation with primary implant insertion for treatment of recalcitrant endophthalmitis and panophthalmitis. Ophthal Plast Reconstr Surg. 2005. 21:220–226.
4. Remulla HD, Rubin PA, Shore JW, et al. Complications of porous spherical orbital implants. Ophthalmology. 1995. 102:586–593.
5. Blaydon SM, Shepler TR, Neuhaus RW, et al. The porous polyethylene (Medpor) spherical orbital implant: a retrospective study of 136 cases. Ophthal Plast Reconstr Surg. 2003. 19:364–371.
6. Tawfik HA, Budin H. Evisceration with primary implant placement in patients with endophthalmitis. Ophthalmology. 2007. 114:1100–1103.
7. Dresner SC, Karesh JW. Primary implant placement with evisceration in patients with endophthalmitis. Ophthalmology. 2000. 107:1661–1664.
8. Merritt K, Shafer JW, Brown SA. Implant site infection rates with porous and dense materials. J Biomed Mater Res. 1979. 13:101–108.
9. Sun DY, Kim YD. Management of exposed porous orbital implants. J Korean Ophthalmol Soc. 2004. 45:1409–1419.
10. Kim JY, Wang SJ, Park CJ, Lee SB. Risk factors leading to enucleation or evisceration in endophthalmitis. J Korean Ophthalmol Soc. 2007. 48:1362–1368.
11. Kim DS, Kim SH, Yoon IH. Exposure incidence of porous orbital implants. J Korean Ophthalmol Soc. 2003. 44:2711–2719.
12. Migliori ME. Enucleation versus evisceration. Curr Opin Ophthalmol. 2002. 13:298–302.
13. Burnstine MA. Primary implant placement with evisceration. Ophthalmology. 2000. 107:1664–1665.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download