Abstract
This prospective observational case series study included 6 eyes of 6 consecutive glaucomatous patients. Each patient underwent trabeculectomy with mitomycin C, and received a 1.25 mg of subconjunctival bevacizumab injection at completion of the trabeculectomy. Study eyes included two with neovascular glaucoma, three with uveitic glaucoma, and one with secondary glaucoma following vitrectomy. All eyes had undergone failed glaucoma laser/surgical treatment or an intraocular surgical procedure. Intraocular pressure (IOP) at the following postoperative visits: preoperative, 1 week, 1 month, 2 months, 3 months, and 6 months, was measured. We also evaluated postoperative bleb findings and complications. IOP measured at each visit was 37.5±14.4 mmHg, 6.2±3.4 mmHg, 8.3±7.2 mmHg, 12.0±4.4 mmHg, 10.8±3.1 mmHg, and 12.2±3.3 mmHg, respectively, for each visit. All eyes had functioning blebs with normal IOP at postoperative 6 months with no additional IOP-lowering medication.
Recent advances in understanding the mechanism of angiogenesis have facilitated the development of new treatment options for neovascular ocular diseases. Neovascularization (NV) occurs as a result of angiogenic stimuli, including the vascular endothelial growth factor (VEGF) [1]. Bevacizumab (Avastin®; Genentech, South San Francisco, CA, USA) is a recombinant humanized monoclonal immunoglobulin G1 antibody directed against VEGF [2]. Bevacizumab was originally granted approval as a first-line therapy in metastatic colorectal cancer [3]. In the field of ophthalmology, encouraging data from clinical studies involving intravitreal bevacizumab injection to treat age-related macular degeneration, severe proliferative diabetic retinopathy, and iris neovascularization have led to trials involving different routes of bevacizumab administration to treat various eye disorders [4-6]. Topical or subconjunctival application of bevacizumab was found to be effective for inhibiting corneal neovascularization in both an experimental rat model and in humans [7, 8].
Modulating the wound healing process to lessen scar formation around the scleral flap plays a crucial role in the success of glaucoma filtering surgery. The use of antimetabolites, such as mitomycin C (MMC) or 5-fluorouracil (5-FU), has been widely advocated for trabeculectomy to modulate fibroblast proliferation. A recent pathological study demonstrated that neutralization of VEGF reduced vascularity and decreased scar formation during wound healing, showing that VEGF strongly influenced scar tissue formation [9, 10]. Jonas et al. [11] also showed the efficacy of intravitreal bevacizumab injection as a supplementary procedure in standard trabeculectomy for eyes showing neovascularization or persistent macular edema. Although encouraging, this report was limited in that the main purpose of intravitreal bevacizumab administration was to control accompanying macular edema or iris neovascularization. Recently, Grewal et al. [12] showed that 11 of 12 eyes (92%) that underwent trabeculectomy exhibited successful intraocular pressure (IOP) control with subconjunctival bevacizumab injection. However, none of the eyes in that study had a history of laser or surgical intervention. We investigated the effect of subconjunctival bevacizumab administration as an adjunct treatment to trabeculectomy in eyes with prior laser or surgical treatment.
A sample of eligible patients was consecutively identified for inclusion in the study from September 2007 to January 2008. Patients with uncontrolled IOP (more than 21 mmHg) on maximal tolerable medical treatment were included. The study eyes consisted of two with neovascular glaucoma (NVG), three with uveitic glaucoma, and one with secondary glaucoma following retinal detachment surgery. All six eyes had a history of failed glaucoma laser treatment or an intraocular surgical procedure (two trabeculectomy, one Ahmed glaucoma drainage implantation, two selective laser trabeculoplasty (SLT), and one multiple intravitreal injections), and two eyes had previous pars plana vitrectomy (PPV) among them. Data collected for analysis included patient demographics, previous ocular or systemic history, slit-lamp evaluation, IOP measurement, postoperative bleb findings, and complications for six months postoperatively. This study was approved by the Institutional Review Board of the HanGil Eye Hospital. Informed patient consent was obtained for the off-label use of bevacizumab from all patients, and all procedures conformed to the tenets of the Declaration of Helsinki.
Fornix- or limbus-based trabeculectomy was performed. Conjunctiva was incised at about 1 mm from the limbus for the fornix-based trabeculectomy, and at about 8 mm from the limbus for the limbus-based trabeculectomy. Tenon's capsule was gently dissected between the capsule and episclera. A lamellar scleral flap was made in a triangular shape, a 0.04% MMC-soaked sponge was applied for 2 minutes beneath the conjunctival and scleral flap, the trabecular and deep peripheral corneal tissues were excised, a peripheral iridectomy was performed, the scleral flap was re-fixed using 2 or 3 stitches of 10-0 nylon interrupted sutures, and the conjunctiva was closed water-tight using a 9-0 Vicryl continuous suture for both the fornix- and the limbus-based trabeculectomy. Upon completion of surgery, 0.2 mL (1.25 mg) bevacizumab was injected subconjunctivally on the opposite side of the bleb. The eye was treated with topical antibiotics (0.5% levofloxacin, Cravit®; Santen, Osaka, Japan), a steroid (1% prednisolone acetate, Pred Forte®; Allergan, Irvine, CA, USA), and cycloplegic eye drops (1% atropine sulfate, Ocu-tropine®; Samil Pharmaceuticals, Seoul, Korea) postoperatively for about a month.
The diagnosis, demographics, preoperative status, and surgical details of each patient are briefly summarized in Table 1. Study eyes included two with neovascular glaucoma, three with uveitic glaucoma, and one with secondary glaucoma following vitrectomy. All eyes had undergone failed glaucoma laser/surgical treatment or an intraocular surgical procedure. As shown in Fig. 1, the IOP measured preoperatively was 37.5±14.4 mmHg; the IOP measured at each visit 1 week, 1 month, 2 months, 3 months, and 6 months postoperatively was.6.2±3.4 mmHg, 8.3±7.2 mmHg, 12.0±4.4 mmHg, 10.8±3.1 mmHg, and 12.2±3.3 mmHg, respectively.
A 75-year-old woman complained of decreased vision and ocular pain in the left eye. The patient was diagnosed with NVG associated with proliferative diabetic retinopathy and was referred by a retinal specialist to our glaucoma clinic. Slit lamp findings included rubeosis iridis, NV of the angles, and severely inflamed conjunctiva, even though the eye had previously undergone multiple sessions of panretinal photocoagulation (PRP), intravitreal triamcinolone acetate (IVTA) injections, and an intravitreal bevacizumab injection. The best-corrected visual acuity (BCVA) was for hand movement. IOP had ranged from 56 to 66 mmHg over a few weeks. Limbus-based trabeculectomy was performed, and IOP measured at postoperative 1 week, 1 month, 2 months, 3 months, and 6 months was 10, 22, 18, 12, and 15 mmHg, respectively. Laser suture lysis was performed 4 weeks after surgery, and digital eyeball massage was recommended. A localized avascular bleb with moderate height was observed six months postoperatively, but the BCVA did not change. No additional IOP-lowering medication was required (Fig. 2A).
A 63-year-old male patient visited our glaucoma clinic diagnosed with NVG associated with ocular ischemic syndrome in the right eye. The eye had undergone trabeculectomy twice at another hospital. Slit lamp findings included pseudoexfoliative material deposition on the lens and the pupillary margin, rubeosis iridis and NV of the angles. The patient had ischemic changes on the retina, and PRP was performed. IOP was not controlled with maximal tolerable medical treatment (MTMT) including oral acetazolamide. BCVA was 20/30, and IOP ranged from 28 to 38 mmHg. Optic disc evaluation revealed increased cup-to-disc (C/D) ratio of 0.9, with inferior neuroretinal rim loss. Fornix-based trabeculectomy was performed. IOP measured at postoperative 1 week, 1 month, 2 months, 3 months, and 6 months was 5, 7, 14, 11, and 8 mmHg, respectively. Microleakage of the conjunctival wound was noted within the first 3 days after surgery, but healed spontaneously without intervention. Laser suture lysis was done at postoperative 3 weeks. A localized avascular bleb with moderate elevation was noted. No additional IOP-lowering medication was required (Fig. 2B).
A 40-year-old man was referred to our glaucoma clinic diagnosed with panuveitic glaucoma in the left eye. The patient was on MTMT, including oral prednisolone and cyclosporin. The eye had previously undergone multiple subtenon injections of triamcinolone acetate and an SLT procedure. The IOP was 27 mmHg, and the BCVA was 20/30. Optic disc evaluation revealed a C/D ratio of 0.7, with inferior neuroretinal rim thinning. Serial visual field examination revealed worsening of the visual field over several months. Fornix-based trabeculectomy was performed. A shallow anterior chamber was noted at postoperative 1 week, and viscoelastic material (Healon GV®; Advanced Medical Optics, Santa Ana, CA, USA) was injected into the anterior chamber. IOP measured at postoperative 1 week, 1 month, 2 months, 3 months, and 6 months was 4, 2, 8, 11, and 14 mmHg, respectively. Cataract surgery was performed 2 months postoperatively due to a progressing posterior subcapsular cataract. A localized avascular bleb with moderate elevation was noted upon final follow-up. The final BCVA was 20/25. No additional IOP-lowering medication was required (Fig. 2C).
A 30-year-old man presented with panuveitic glaucoma due to medically uncontrollable IOP in the right eye. The eye had previously undergone PPV and Ahmed glaucoma drainage device implantation two years prior. Multiple IVTA injections had been administered previously to control the uveitis. BCVA was 20/60 due to the cataract, and the IOP was 32 mmHg on MTMT. Optic disc evaluation revealed a C/D ratio of 0.8, with localized notching in the inferior neuroretinal rim. A dense arcuate scotoma was identified during a Humphrey visual field examination. Two-sited phacotrabeculectomy was performed. After the cataract was extracted and an intraocular lens (IOL) implanted, fornix-based trabeculectomy was performed in the superior area. IOP measured at postoperative 1 week, 1 month, 2 months, 3 months, and 6 months was 4, 5, 6, 5, and 8 mmHg, respectively. Diffuse and low bleb formation with no serious postoperative complications was noted during the follow-up period. Final BCVA was 20/30. No additional IOP-lowering medication was required (Fig. 2D).
A 25-year-old man presented with increased IOP in the right eye. The patient had cataract extraction and an IOL implantation due to posterior subcapsular cataract one year ago. Subsequently, the eye underwent PPV with encircling buckling due to retinal detachment. The IOP was uncontrollable with MTMT after surgery. SLT had been repeated twice with at an interval of 1 month, but the effect was temporary. IOP was 32 mmHg at one month after the second SLT procedure, and the BCVA was 20/20. Slit lamp findings revealed normal open-angle on gonioscopy, and the C/D ratio was 0.6 with concentric cup enlargement. Limbus-based trabeculectomy was performed. During the surgery, the surgeon (JC) found that there was severe adhesion between the scleral buckle and overlying conjunctiva behind the conjunctival incision line. IOP measured at postoperative 1 week, 1 month, 2 months, 3 months, and 6 months was 11, 10, 14, 12, and 14 mmHg, respectively. A localized avascular bleb with moderate elevation was noted, but no serious postoperative complications were noted. The final BCVA was 20/20. No additional IOP-lowering medication was required (Fig. 2E).
A 73-year-old man with anterior uveitis had an IOP ranging from 17 to 30 mmHg in the left eye. Seven months ago, the patient underwent trabeculectomy in the superonasal area, following the bleb needling procedure with multiple 5-FU injections, upon uveitic glaucoma diagnosis. Worsening optic disc appearance and visual field changes were noted. The BCVA was 20/50. Fornix-based trabeculectomy was performed in the superotemporal area. Ocular hypotony with mild choroidal effusion persisted for about one month after surgery. The bleb was diffuse and elevated with no conjunctival leakage. IOP measured at one week and one month was 3 and 4 mmHg, respectively. Wound revision with additional suture of the scleral flap was performed at postoperative one month. During the revision procedure, the surgeon (JC) found that there was no blood vessel growth at either wound adhesion around the scleral flap (Fig. 2F). IOP normalized to 12 mmHg two months after the revision, and the choroidal effusion resolved. Diffuse and low bleb with less marked vascularization was noted postoperatively. The final BCVA was 20/40. No additional IOP-lowering medication was required.
Conventional trabeculectomy is reported to be less successful in eyes that have undergone failed glaucoma surgery or have a disease with poor prognosis (i.e. NVG or uveitic glaucoma), when compared to the eyes with primary disease, probably due to the aggressive wound healing process associated with excessive inflammation, adhesion, or angiogenesis [13]. The low success rates of filtering surgery in these eyes have led to investigation of the adjunct trabeculectomy regimen. The use of antimetabolites (MMC or 5-FU), amniotic membrane, or even photodynamic therapy has been advocated to enhance the surgical outcomes [14-16].
Angiogenesis, the process of new blood vessel formation, is a key element in the proliferative phase of wound healing, supplying oxygen and nutrients to support the rapid growth of cell-mediated repair. Wilgus et al. [9] reported that VEGF promoted angiogenesis and scar formation in early fetal skin and that a VEGF blockade influenced the organization of scar tissue. VEGF neutralization not only reduced the amount of scar tissue formed, but also improved the quality of the scar tissue that did form by shifting the collagen fibril distribution to a state more closely resembling normal skin in a mouse model. In a mouse model of bleomycin-induced pneumopathy, transfection of the VEGF receptor (sflt-1) gene attenuated pulmonary fibrosis, suggesting that anti-VEGF treatment might be used as an antifibrotic therapy [17].
The most important cause of persistent IOP elevation after trabeculectomy is unduly marked or persistent inflammation, followed by deposition of fibrous tissue, which prevents the formation of an adequately draining bleb [18]. Modifying the wound healing process of the scleral flap upon trabeculectomy could enhance the surgical outcome. The topical and systemic applications of bevacizumab significantly inhibit both inflammation-induced angiogenesis and lymphangiogenesis in the cornea [19]. Reducing the amount of cytokines (e.g., fibroblast growth factor, VEGF) released from the vessels to the site of injury by blocking angiogenesis with bevacizumab may indirectly render the scleral flap less adherent to its original site during the immediate postoperative period, allowing more fluid to drain through the flap. Another possible explanation could be that bevacizumab itself acts on the scleral flap's scar formation directly through fibroblast modulation [9]. Wu et al. [20] recently demonstrated VEGF's ability to induce keloid fibroblast proliferation and suggested the presence of functional VEGF receptors on fibroblasts. The presence of VEGF receptors on fibroblasts is also supported by a study demonstrating expression of VEGF receptor-2 in stromal cells during wound repair in vivo [21]. A recent study showed that postoperative subconjunctival injection of bevacizumab was associated with improved trabeculectomy bleb survival in the rabbit model, suggesting bevacizumab may be a useful agent for improving the success rate and limiting scar tissue formation after trabeculectomy [22].
We found that the IOPs of all patients were within the normal range during the 6 month follow-up period. Postoperative complications in our study included early hypotony with IOP <5 mmHg (three eyes), cataract development (one eye), and microleakage of the conjunctival wound (one eye). A bleb revision procedure was performed one month after trabeculectomy in Case 6; neither vessel formation nor adhesion around the scleral flap was observed. Alternatively, relatively higher incidences of early hypotony I our series of patients and no vessel growth observed in Case 6 may hold clues to the potential of bevacizumab to modify the wound healing process following trabeculectomy. However, uncertain was the association of subconjunctivally injected bevacizumab with these surgical outcomes in our series of patients.
In a previous study, disintegration of the corneal epithelium and progression of stromal thinning have been reported in an eye undergoing topical bevacizumab application for four weeks, suggesting that treatment may be related to adhesion between the epithelium and the basement membranes or inhibit the normal wound healing process [9]. While the inhibition of angiogenesis could play a beneficial role in the scleral flap healing process, also possible is that interrupted wound healing may dispose the conjunctival incision to postoperative leakage in trabeculectomy. Precise surgical skill for watertight conjunctival closure is warranted if subconjunctival bevacizumab is used as an adjunct regimen to trabeculectomy.
Our study has some limitations. Separating the effect of bevacizumab from that of concomitantly applied MMC on the wound healing process is difficult, as this study has taken the form of a small case series study design rather than a case-controlled one. Hence, suggesting that the high success rate in this study is wholly dedicated to the adjuvant use of subconjunctival bevacizumab would be inappropriate, as would be claiming that one drug has more potency in wound healing process than the others. The rather small number of subjects and short follow-up period for glaucoma are also major limitations. The efficacy and safety should be tested in the further case-controlled studies.
In summary, our report suggests that subconjunctival bevacizumab administration may be an effective and safe adjunct regimen to trabeculectomy in eyes with refractory glaucoma. While the blockage of angiogenesis and possible fibroblast modulation with anti-VEGF agent may provide some benefits for glaucoma filtering surgery, adverse complications related to the delayed wound healing process may also be associated. Basic research and randomized, controlled long-term clinical studies are required to provide further knowledge regarding the mechanism and application of bevacizumab as an adjunct treatment to trabeculectomy.
Figures and Tables
Fig. 1
Intraocular pressure (IOP) changes following trabeculectomy plus subconjunctival bevacizumab administration.

Fig. 2
Representative postoperative slit lamp photographs. (A) Case 1. Localized avascular bleb formation in an eye with neovascular glaucoma (NVG). (B) Case 2. Localized bleb formation in the superior area in an eye with NVG. Note the presence of previously failed trabeculectomy in the superonasal and super-otemporal areas. (C) Case 3. Localized avascular bleb formation in an eye with uveitic glaucoma. (D) Case 4. Diffuse and low bleb formation in an eye with uveitic glaucoma. Note the presence of a previously failed Ahmed drainage device in the superotemporal area. (E) Case 5. Localized avascular bleb formation in an eye with secondary glaucoma following retinal detachment surgery. Note the presence of the anchoring suture of the scleral buckle in the superotemporal subconjunctival area. (F) Case 6. Wound revision at 1 month after trabeculectomy in Case 6 revealed the absence of blood vessel formation or wound adhesion around the scleral flap.
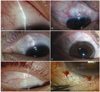
Table 1
Summary of patients treated with subconjunctival bevacizumab as an adjunct to trabeculectomy

TRAB=trabeculectomy; IOP=intraocular pressure; LSL=laser suture lysis; MED=anti-glaucomatous medication; NVG=neovascular glaucoma; PDR=proliferative diabetic retinopathy; PRP=panretinal photocoagulation; IVTA=intravitreal triamcinolone acetate; IV=intravitreal; OIS=ocular ischemic syndrome; PEX= pseudoexfoliation; TA=triamcinolone acetate; PPV=pars plana vitrectomy; GDDI=glaucoma drainage device implantation; RD=retinal detachment; EB=encircling buckle; SLT=selective laser trabeculoplasty; 5-FU=5-fluorouracil.
References
1. Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004. 82:557–563.
2. Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997. 57:4593–4599.
3. Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005. 23:792–799.
4. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006. 26:495–511.
5. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006. 113:1695.e1–1695.e15.
6. Wakabayashi T, Oshima Y, Sakaguchi H, et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology. 2008. 115:1571–1580.
7. Manzano RP, Peyman GA, Khan P, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). Br J Ophthalmol. 2007. 91:804–807.
8. Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008. 115:e33–e38.
9. Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008. 88:579–590.
10. Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009. 50:5217–5225.
11. Jonas JB, Spandau UH, Schlichtenbrede F. Intravitreal bevacizumab for filtering surgery. Ophthalmic Res. 2007. 39:121–122.
12. Grewal DS, Jain R, Kumar H, Grewal SP. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy: a pilot study. Ophthalmology. 2008. 115:2141–2145.
13. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003. 48:314–346.
14. Jordan JF, Diestelhorst M, Grisanti S, Krieglstein GK. Photodynamic modulation of wound healing in glaucoma filtration surgery. Br J Ophthalmol. 2003. 87:870–875.
15. Lu DW, Chang CJ, Chiang CH, et al. Wound modulation after trabeculectomy by different formulations of antimetabolites in rabbits. J Ocul Pharmacol Ther. 2000. 16:529–538.
16. Demir T, Turgut B, Celiker U, et al. Effects of octreotide acetate and amniotic membrane on wound healing in experimental glaucoma surgery. Doc Ophthalmol. 2003. 107:87–92.
17. Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007. 29:976–985.
18. Vote B, Fuller JR, Bevin TH, Molteno AC. Systemic anti-inflammatory fibrosis suppression in threatened trabeculectomy failure. Clin Experiment Ophthalmol. 2004. 32:81–86.
19. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007. 48:2545–2552.
20. Wu WS, Wang FS, Yang KD, et al. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. 2006. 126:1264–1271.
21. Zhang N, Fang Z, Contag PR, et al. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood. 2004. 103:617–626.
22. Memarzadeh F, Varma R, Lin LT, et al. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009. 50:3233–3237.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download