Abstract
Purpose
To investigate the relationship between vascular endothelial growth factor (VEGF) and the cancer stem cell-vascular niche complex in human retinoblastoma tissue.
Methods
Six human retinoblastoma specimens primarily enucleated for Reese-Ellsworth classification stage 5a were stained to detect cancer stem cell markers, including ABCG2 for the stem cell marker and MCM2 for the neural stem cell marker, as well as to detect VEGF for the angiogenic cytokine. Using immunofluorescence, the expression of these proteins was analyzed, and their relative locations noted.
Results
In non-neoplastic retina of tumor-bearing eyes, ABCG2 and MCM2 were sporadically expressed in the ganglion cell layer and the inner nuclear layer, whereas VEGF was sporadically expressed in inner retina where retinal vessels are abundantly distributed. In the tumor, ABCG2 was strongly expressed out of Wintersteiner rosettes, whereas MCM2 and VEGF were strongly stained in the rosettes. Interestingly, the outer portion of the rosettes was positive for MCM2, and the inner portion of the rosettes was positive for VEGF.
Conclusions
Our data demonstrated that MCM2 and VEGF are strongly expressed in the rosettes of the tumor, which were far from the area of ABCG2-positive cells. Although VEGF might not directly contribute to the cancer stem cell-vascular niche complex, it could play some role in the differentiation of tumor cells to build up the rosettes.
Cancer stem cells (CSCs) have been introduced and investigated as a new hypothesis of tumorigenesis in recent years [1, 2]. In this theory, a population of cells with stem cell- like characteristics, i.e. self-renewal, multipotency, and tumor initiation capability, exists in tumors. This population gives rise to the bulk of the tumor cells with more differentiated phenotypes. CSCs could be helpful not only in understanding the pathogenesis of the cancer, but also in the treatment of the cancer itself. CSCs were regarded as the key component of chemoresistance of cancers, and it should be the target for drug development. Recent study revealed that selective targeting of CSCs is possible [3].
CSCs, like normal stem cells, are believed to exist within a protective perivascular niche, but can escape from the niche control. The presence of a perivascular niche was proved in the hematopoietic system, as well as in brain tumors [4, 5]. Calabrese et al. [5] suggest that CSCs in brain tumors, similar to neural stem cells, reside in vascular niches that are important targets for therapeutic approaches. A few orthotopic xenograft studies in brain tumors suggest that CSCs secrete angiogenic factors, such as vascular endothelial growth factor (VEGF), that promote the recruitment and formation of tumor blood vessels [5, 6].
Retinoblastoma is the most common intraocular childhood tumor [7]. Moreover, retinoblastoma is believed to be a good model in which to search for a tumor cell of origin and to understand the molecular pathogenesis of malignant tumors [8, 9]. Recent studies demonstrate that a small proportion of human and mouse retinoblastoma cell populations express cancer stem cell markers (including ABCG2) and neural stem cell markers (including MCM2) [10, 11]. Zhong et al. [12] demonstrated the presence of tumorigenic retinal stem-like cells that contribute to tumorigenesis in human retinoblastoma.
Without regard to relation with cancer stem cells or a perivascular niche, VEGF has been studied as an important angiogenic growth factor in retinoblastoma [13, 14]. In recent studies, anti-angiogenic therapy targeting VEGF has been proposed as a promising new treatment strategy of retinoblastoma [15-17].
In this study, we investigated the expression of ABCG2 and MCM2 proteins in human retinoblastoma tissues and the correlation of their expression with VEGF proteins, which may be related to the CSCs-perivascular niche complex. The purpose of this study was to evaluate the relation between VEGF and the cancer stem cell-vascular niche complex in human retinoblastoma tissue.
Six patients who were diagnosed as having unilateral highly progressive large retinoblastoma, group 5a in Reese-Ellsworth classification, were included in this study. All patients underwent enucleation without chemotherapy or focal therapies. All human retinoblastoma tissue samples were obtained with informed consent and in accordance with the tenets of the Declaration of Helsinki.
For the detection of ABCG2 protein, mouse monoclonal anti-ABCG2 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used. Primary goat polyclonal anti-MCM2 antibody (1:200, Santa Cruz Biotechnology) and primary rabbit polyclonal anti-VEGF antibody (1:100, Santa Cruz Biotechnology) were also used for staining.
Paraffin-embedded enucleated eyes were sectioned into 4-µm sections. Sections of archival human specimens were rehydrated with xylene and graded alcohols. After washing in running water for five minutes, antigen retrieval was performed by placing slides in 10 mM sodium citrate buffer (pH 6.0), heated at 120℃ for 10 minutes. The slides and buffer were then cooled to room temperature for 30 minutes. After washing with phosphate buffered saline (PBS), the slides were incubated with 0.2% Tripton X-100 for 15 minutes. After three rinses in PBS for five minutes each, sections were incubated with blocking solution for 30 minutes, followed by incubation with primary antibodies: five hours for ABCG2 and VEGF and two hours for MCM2. All sections were incubated for two hours with secondary antibody, followed by mounting.
ABCG2 and MCM2 were sporadically expressed in the ganglion cell layer, the inner nuclear layer, and the photoreceptor layer in the non-neoplastic portion of the retina of the tumor-bearing eye. Expression of these markers seemed not to be related with retinal vasculature (Fig. 1A and 1B).
VEGF was sporadically expressed in the inner retina where retinal vessels are abundantly distributed (Fig. 1C).
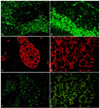
ABCG2 was strongly expressed in the Wintersteiner rosettes or tumor lobules. In the rosettes or lobules, sporadic expression of ABCG2 was observed and not related to tumor vasculature. Most of ABCG2-stained cells were adjacent to the rosettes (Fig. 2A and 2B).
MCM2 was strongly positive in the rosettes, especially in the outer portion of the rosettes (Fig. 2C and 2D). Within the lobules of the tumor, MCM2 was expressed around the blood vessels.
VEGF was expressed in the rosettes or tumor lobules, similar to MCM2. But unlike MCM2, VEGF was strongly expressed in the inner portion of the rosettes (Fig. 2E and 2F). In the lobules of retinoblastoma, VEGF was also sporadically expressed, but not directly associated with tumor vessels.
Evidence suggests that CSCs exist in human retinoblastoma [10, 11, 18]. Seigel et al. [18] observed the presence of a small population which express a cancer stem cell marker (ABCG2) and a neural stem cell marker (MCM2) in tumors from transgenic mice, human retinoblastoma cell lines, and human retinoblastomas. ABCG2 is a half ATP-binding cassette transporter in the G2 subfamily that has been utilized to identify stem cells in several types of cancer. ABCG2 is also related to a resistance to a wide variety of anticancer drugs [19]. MCM2 is one of six members of the family of minichromosome maintenance proteins involved in initiating DNA replication [20]. MCM2 is a proven marker of neural stem cells and is known to be related to the aggressiveness of neuroectodermal tumors and retinoblastoma [11, 21].
In this study, the expression of ABCG2 and MCM2 in human archival retinoblastoma tumors and the non-neoplastic retina in the same eye was observed by immunofluorescence staining. Concurrent with previous studies, MCM2 positivity was observed surrounding blood vessels in both well- and poorly-differentiated tumor tissues. MCM2 was strongly positive in tumor rosettes. In normal retinas, MCM2 positivity was observed in the ganglion cell layer, inner nuclear layer, and photoreceptor layer. The ABCG2 staining pattern was similar in the normal retina. In retinoblastoma tumor tissues, on the other hand, ABCG2 positivity was observed outside of the tumor rosettes and lobules. This result differed from previous reports. With our data, ABCG2 and MCM2 stained different portions of the retinoblastoma tumor tissue.
According to the cancer stem cell hypothesis, the cancer stem cell-vascular niche is an important aspect in understanding CSCs and in differentiating them from normal stem cells. Stem cells reside in "niches", defined as physical spaces in the tissue used to maintain homeostasis [4]. CSCs might escape from normal niche control, and the dysregulated stem cell division may give rise to turmor- igenesis. CSCs secrete angiogenic factors, such as VEGF, that promote the recruitment and formation of tumor vasculature [5, 6].
VEGF had been studied as an important regulator of physiologic and pathologic angiogenesis before the concept of CSCs emerged. VEGF expression in retinoblastoma has also been studied because retinoblastomas are highly vascularized tumors that are dependent on angiogen- esis [13, 14, 22]. Recent studies have revealed that VEGF-targeted treatments suppress tumor angiogenesis and growth significantly in retinoblastomas. In these studies, the anti- angiogenic effect by blocking VEGF was considered the most important mode of action [15-17]. Until now, the mechanism of action with anti-VEGF agents has not been fully understood [23].
In this study, we speculate that VEGF is related to CSCs or the CSCs-perivascular niche complex. VEGF is known to be secreted from neoplastic cells and influences endothelial cells as paracrine mediators, but the relationship between VEGF and CSCs in retinoblastomas is unknown. We stained the VEGF protein in retinoblastoma tissues to locate the expression and to compare it to that of ABCG2 and MCM2. Consistent with previous studies, VEGF immunoreactivity was observed sporadically at the inner retina where there is abundant retinal vasculature in the normal retina. However, this staining was weak and not directly related to retinal vasculature. In retinoblastoma tumor tissues, VEGF expression was observed in the rosettes or tumor lobules, similar to MCM2 expression. Unlike MCM2, strong VEGF expressed was observed in the inner portion of the rosettes. We have no immediate explanation for the difference in the distribution of these proteins. One possible reason that VEGF expression is observed at the inner portion of the rosettes is that it is a secretary protein. The lobules of the retinoblastoma also showed sporadic VEGF positivity but that was not directly associated with tumor vessels. If CSCs or the CSCs-perivascular niche have pivotal roles in VEGF secretion, VEGF immunopositivity must be observed in similar locations as CSC markers. Similarly, if VEGF directly contributes to the CSCs-perivascular niche complex, then VEGF must be located around the tumor vasculature. However, our data show that VEGF expression is not related to ABCG2 expression or tumor vasculature. Instead, VEGF expression was similar to that of MCM2 except with specific topographical distribution. Our study suggests that, although VEGF might not directly contribute to the CSC-perivascular niche complex, it could play some role in the differentiation of tumor cells and in rosette formation.
Figures and Tables
Fig. 1
ABCG2, MCM2, and VEGF expression in the non-neoplastic retina of the human retinoblastoma eye. (A) ABCG2 was sporadically expressed in the ganglion cell layer and the inner nuclear layer. Original magnification ×20. (B) MCM2 positivity was observed in GCL and INL, similar to ABCG2. Original magnification ×20. (C) VEGF was also sporadically expressed in the inner retina. Original magnification ×20. VEFG=vascular endothelial growth factor; GCL=ganglion cell layer; IPL=inner plexiform layer; INL=inner nuclear layer; OPL=outer plexiform layer; ONL=outer nuclear layer.
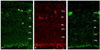
Fig. 2
ABCG2, MCM2, and VEGF expression in retinoblastoma tumors. (A and B) ABCG2 was expressed outside of the Wintersteiner rosettes or tumor lobules. (C and D) MCM2 was strongly expressed in the rosettes and lobules, especially in the outer portion of the rosettes. (E and F) VEGF positivity was also observed in the rosettes of lobules, specifically in the inner portion of the rosettes. Original magnification ×20 with A, C, and E. Original magnification ×40 with B, D, and F.
Acknowledgements
We thank Mr. Hyung On Jun, Mr. Chang Sik Cho, and Ms. Esther Yang for their technical assistance. This study was supported by a grant from the National R&D program for cancer control of the Ministry of Health & Welfare, Republic of Korea (0820170) and a grant (KRF-2008-331-E00257) from the Korea Research Foundation.
References
1. Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007. 58:267–284.
2. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001. 414:105–111.
3. Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006. 441:475–482.
4. Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008. 8:290–301.
5. Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007. 11:69–82.
6. Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006. 66:7843–7848.
7. Abramson DH. Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2005. 46:2683–2691.
8. Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005. 5:91–101.
9. Nork TM, Schwartz TL, Doshi HM, Millecchia LL. Retinoblastoma. Cell of origin. Arch Ophthalmol. 1995. 113:791–802.
10. Seigel GM, Hackam AS, Ganguly A, et al. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007. 13:823–832.
11. Mohan A, Kandalam M, Ramkumar HL, et al. Stem cell markers: ABCG2 and MCM2 expression in retinoblastoma. Br J Ophthalmol. 2006. 90:889–893.
12. Zhong X, Li Y, Peng F, et al. Identification of tumorigenic retinal stem-like cells in human solid retinoblastomas. Int J Cancer. 2007. 121:2125–2131.
13. Kvanta A, Steen B, Seregard S. Expression of vascular endothelial growth factor (VEGF) in retinoblastoma but not in posterior uveal melanoma. Exp Eye Res. 1996. 63:511–518.
14. Stitt AW, Simpson DA, Boocock C, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumours. J Pathol. 1998. 186:306–312.
15. Apte RS, Harbour JW. Inhibiting angiogenesis in retinoblastoma. Ophthalmic Res. 2007. 39:188–190.
16. Jia RB, Zhang P, Zhou YX, et al. VEGF-targeted RNA interference suppresses angiogenesis and tumor growth of retinoblastoma. Ophthalmic Res. 2007. 39:108–115.
17. Lee SY, Kim DK, Cho JH, et al. Inhibitory effect of bevacizumab on the angiogenesis and growth of retinoblastoma. Arch Ophthalmol. 2008. 126:953–958.
18. Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005. 11:729–737.
19. Litman T, Brangi M, Hudson E, et al. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2). J Cell Sci. 2000. 113:2011–2021.
20. Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004. 24:1726–1733.
21. Schlesinger HR, Rorke L, Jamieson R, Hummeler K. Neuronal properties of neuroectodermal tumors in vitro. Cancer Res. 1981. 41:2573–2575.
22. Pe'er J, Neufeld M, Baras M, et al. Rubeosis iridis in retinoblastoma. Histologic findings and the possible role of vascular endothelial growth factor in its induction. Ophthalmology. 1997. 104:1251–1258.
23. Grothey A, Ellis LM. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer J. 2008. 14:170–177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download