Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Fig. 1
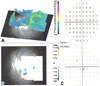
Fig. 2

Table 2

F=female; GDx VCC=GDx VCC scanning laser polarimeter; HRT II=Heidelberg retinal tomograph II confocal scanning laser ophthalmoscopy; Lt=left; M=male; Rt=right; RTA=retinal thickness analyzer; Stratus OCT=Stratus OCT optical coherence tomography; Values given as mean ±standard deviation; *One-way analysis of variance.
Table 5

NFI=nerve fiber indicator; PFAT=perifoveal abnormally thin area; PFMT=perifoveal minimum thickness; PFSIA=perifoveal superior/inferior asymmetry; PPAT=posterior pole abnormally thin area; PPMT=posterior pole minimum thickness; PPNT=posterior pole number of thin clusters; PPPD=posterior pole pattern deviation; PPSIA=posterior pole superior/inferior asymmetry; TSNIT=temporal-superior-nasal-inferior-temporal; Values given as correlation coefficient (p-value); Statistical analysis was performed using the Pearson correlation coefficient; *p<0.05.
Table 6

Avg. Thick=average thickness; Iavg=inferior average; Imax=inferior maximum; PFAT=perifoveal abnormally thin area; PFMT=perifoveal minimum thickness; PFSIA=perifoveal superior/inferior asymmetry; PPAT=posterior pole abnormally thin area; PPMT=posterior pole minimum thickness; PPNT=posterior pole number of thin clusters; PPPD=posterior pole pattern deviation; PPSIA=posterior pole superior/inferior asymmetry; Savg=superior average; Smax=superior maximum; Values given as correlation coefficient (p-value); Statistical analysis was performed using the Pearson correlation coefficient; *p<0.05.
Table 7

PFAT=perifoveal abnormally thin area; PFMT=perifoveal minimum thickness; PFSIA=perifoveal superior/inferior asymmetry; PPAT=posterior pole abnormally thin area; PPMT=posterior pole minimum thickness; PPNT=posterior pole number of thin clusters; PPPD=posterior pole pattern deviation; PPSIA=posterior pole superior/inferior asymmetry; RNFL=retinal nerve fiber layer; Values given as correlation coefficient (p-value); Statistical analysis was performed using the Pearson correlation coefficient; *p<0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download