Abstract
Purpose
To present a case of inflammatory myofibroblastic tumor which was manifested as an idiopathic subretinal mass without underlying pathology.
Methods
The subretinal mass was surgically excised and evaluated histopathologically. Fluorescein angiography and optical coherence tomography were performed pre- and post-operatively.
Results
The mass was histologically composed of lymphoplasma cell infiltration and fibrous proliferation without microorganisms or malignant cells. Immunohistochemistry for smooth muscle actin revealed myofibroblasts as a major cellular component. Preoperative optical coherent tomography showed that the lesion was contiguous to the retina while inducing foveal detachment. Postoperatively, the fovea was attached with visual recovery, and the subretinal lesion did not recur during the follow up.
Inflammatory pseudotumors presently include several biological processes such as benign, reactive conditions, most often associated with an infectious etiology, as well as neoplasms of dendritic cells or myofibroblasts.1-3 The lesions are composed of varying mixtures of acute inflammation, plasma cells, fibroblasts, and myofibroblasts. When the pathology is characterized by spindle cell proliferations composed of myofibroblasts, they are termed "inflammatory myofibroblastic tumors."1
Inflammatory myofibroblastic tumors have been reported in numerous anatomic sites. Although very rare, they can develop in the eye with different clinical manifestations such as ciliary body mass, uveitis, iris infiltration, narrow angle glaucoma, scleritis, keratitis, or masses filling the vitreous cavity.
We report a case of an inflammatory myofibroblastic tumor which presented as a solitary subretinal mass without underlying pathology. Informed consent was obtained from the patient.
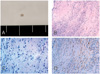
A 35-year-old man presented with a 2-month history of decreased vision in his left eye. Approximately 2 months before the examination, the patient noted a sudden onset of decreased vision in the left eye, and the poor vision remained until the examination. Past ocular and medical histories were unremarkable, and the patient was otherwise in good health. On examination, the best-corrected visual acuities were 20/20 in his right eye and 20/40 in his left. There were no refractive errors in either eye. An anterior segment examination was unremarkable, and intraocular pressures were 15 mmHg in both eyes. There were no anterior chamber or vitreous cells in either eye. A fundus examination of the left eye revealed a well-defined, white subretinal mass (Fig. 1A). The lesion was approximately one disc diameter in size and one and a half disc diameters superior to the foveal center, accompanying the subretinal fluid. Optical coherence tomography showed that the mass was contiguous to the retina, and that the subretinal fluid extended to the foveal center (Fig. 1E). Results of systemic and serologic evaluations were normal. Fluorescence angiography (FA) revealed hyperfluorescence in both early and late frames (Fig. 1C). The patient underwent a pars plana vitrectomy to excise the mass and attach the fovea. Vitreous was removed after induction of posterior hyaloid detachment, and then a balanced salt solution was injected subretinally with a 40 gauge needle, making a retinal detachment of the posterior pole. The adhesion between the mass and overlying retina was relieved by subretinal microscissors following a retinotomy superior to the mass. The adhesion between the mass and the choroid was also relieved and the mass was removed through the retinotomy using subretinal forceps. No significant subretinal bleeding occurred during the procedures. Fluid-air exchange was performed, followed by an endolaser around the retinotomy site. At the end of the vitrectomy, SF6 gas was injected intravitreally. The excised lesion was observed and sectioned. The lesion was 1×1 mm-sized, firm, round, yellowish mass (Fig. 2A). The mass was evaluated by hematoxylin-eosin and immunohistochemical staining including α-smooth muscle actin as a smooth muscle marker and S-100 as a neurogenic tumor marker. The pathological examination of the excised subretinal mass revealed a spindle cell proliferation with a lymphoplasma cell infiltration. Fibrosis was also observed (Fig. 2B, 2C). Immunohistochemistry showed that the spindle cells were positive for α-smooth muscle actin, but negative for S-100, indicating they were mainly myofibroblasts (Fig. 2D). Neither eosinophils nor multinucleated giant cells were seen. In addition, no microorganisms or malignant cells were seen, and few vascular channels were observed. One month after surgery, visual acuity improved to 20/20. The fundus examination showed chorioretinal atrophy in the excision site, but no abnormal finding in the fovea (Fig. 1B). FA and optical coherent tomography showed no subretinal fluid in the macular area (Fig. 1D, 1F). Functional and anatomic outcomes were maintained for a period of 15 months without any sign of a recurrence.
The subretinal mass in the patient showed chronic inflammation, which was characterized by lymphoplasma cell infiltration together with the proliferation of collagenous tissue in the absence of granulomatous inflammation. The spindle cells were mainly myofibroblasts that were positive for the smooth muscle marker. No microorganisms or malignant cells were observed. These findings were compatible with those of inflammatory myofibroblastic tumors.
The differential diagnosis of a solitary subretinal mass includes reactive lymphoid hyperplasias, subretinal fibrosis associated with uveitis, choroidal neovascular membranes, neoplasms, and parasitic or fungal infections such as toxoplasma, toxocara, and ocular histoplasmosis syndrome. The etiology of a parasitic or fungal infection was excluded because no obvious retinal inflammation or necrosis was observed in the pathology. In addition, no evidence of eosinophilia or granulomatous inflammation was seen in either ocular and systemic examinations. The patient had not lived nor traveled to an endemic area of histoplasmosis, and the serologic tests for histoplasmosis, syphilis, toxocara, and toxoplasma antibodies were all negative. Subretinal fibrosis associated with uveitis was also unlikely in that there was no inflammation in the anterior segment and vitreous cavity, and the patient had no history of uveitis or other ocular inflammation. A diagnosis of choroidal neovascularization was also excluded because the patient was young and not myopic, and the subretinal lesion showed few vascular channels.
Intraocular inflammatory pseudotumors were previously described as various manifestations such as a ciliary body mass, uveitis, iris infiltration, narrow angle glaucoma,4 scleritis, keratitis,2 subretinal lesions in age-related macular degenerations,5 or masses filling the vitreous cavity.3 However, this case is unique in that it was located in the subretinal space without any underlying pathology. The mass also accompanied the surrounding subretinal fluid, causing subfoveal detachment, which was confirmed by optical coherent tomography. Heidenkummer & Kampik5 reported the presence of myofibroblasts in disciform subretinal lesions extracted from eyes of patients with age-related macular degeneration (AMD). However, the patient in this case differs from theirs in that he had no AMD pre-operatively and the lesion was a solitary, round mass contiguous to the retina. Moreover, the subretinal lesion in this case showed few vascular channels in contrast to the lesion in Heidenkummer & Kampik's case with large hematoma and feeder vessels that were characteristic of AMD.5
The etiology of an inflammatory pseudotumor, or inflammatory myofibroblastic tumor, remains unclear, even though a number of theories have been proposed; including infectious agents, tumor-associated factors, and cytokines.1 Infectious agents, such as EBV, were identified in some inflammatory myofibroblastic tumors by in situ hybridization and immunohistochemistry for the presence of the virus.1 The presence of clonal abnormalities of the inflammatory myofibroblastic tumors was confirmed by immunohistochemistry, various cell marker studies, and chromosomal studies.3 Therefore, in order to fully understand the etiology of a spindle cell proliferation, it may be necessary to perform these ancillary studies. Though this patient showed no recurrence during the follow-up period, inflammatory myofibroblastic tumors have been known to recur, thus continuous follow-up are needed.
In summary, we report a case of an inflammatory myofibroblastic tumor, presented as a solitary subretinal mass and excised surgically. This case suggests that inflammatory myofibroblastic tumors may be included in the differential diagnosis of subretinal masses.
Figures and Tables
Fig. 1
Clinical findings pre- (A, C, E) and post-operatively (B, D, F). (A) Color fundus photograph shows a well-defined, white subretinal mass. (B) Chorioretinal atrophy developed in the excision site. (C) Fluorescence angiography shows tumor staining with a distinct border in the late frames. (D) Late phase of fluorescence angiogram shows choroidal atrophy at the excision site. (E) Optical coherence tomography shows that the lesion is contiguous to the retina (above), while inducing macular detachment (below). (F) Neurosensory retinal detachment disappeared in both the excision site (above) and macula (below).
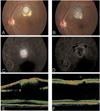
Fig. 2
(A) Gross photograph of the subretinal lesion shows a 1×1 mm -sized, firm, round, and yellowish tumor. (B) Pathology shows both lymphoplasma cell infiltration and fibrous proliferation (hematoxylin and eosin, ×100). (C) Lymphoplasma cells were infiltrated, presenting chronic nongranulomatous inflammation (hematoxylin-eosin, ×400). (D) Immunohistochemistry for α-smooth muscle actin, a marker for smooth muscle differentiation (×200). The majority of cells in the fibrous proliferation are myofibroblasts, which are α-smooth muscle actin-positive (brown).
References
1. Neuhauser TS, Derringer GA, Thomson LD, et al. Splenic Inflammatory Myofibroblastic Tumor (Inflammatory Pseudotumor): A Clinicopathologic and Immunophenotypic Study of 12 Cases. Arch Pathol Lab Med. 2001. 125:379–385.
2. Uy HS, Nguyen QD, Arbour J, et al. Sclerosing inflammatory pseudotumor of the eye. Arch Ophthalmol. 2001. 119:603–607.
3. O'Malley DP, Poulos C, Czader M, et al. Intraocular inflammatory myofibroblastic tumor with ALK overexpression. Arch Pathol Lab Med. 2004. 128:e5–e7.
4. Bernardino CR, Davidson RS, Maus M, Spaeth GL. Angle-closure glaucoma in association with orbital pseudotumor. Ophthalmology. 2001. 108:1603–1606.
5. Heidenkummer HP, Kampik A. Surgical extraction of subretinal pseudotumors in age related macular degeneration. Clinical, morphologic and immunohistochemical results. Ophthalmologe. 1995. 92:631–639.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download